October 17, 2017 — The American College of Cardiology and the American Heart Association recently released updated ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
October 17, 2017 — BioVentrix Inc. recently announced enrollment of the first patient in the U.S. arm of the ALIVE ...

Three-dimensional (3-D) printed anatomic models created from a patient’s computed tomography (CT), magnetic resonance ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
Bill Lombardi, M.D., director of complex coronary artery interventions at the University of Washington, discusses the ...
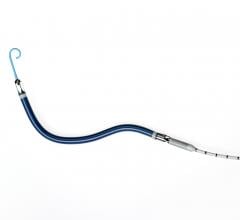
October 5, 2017 — BTG plc announced it has acquired Roxwood Medical, provider of advanced cardiovascular specialty ...
October 4, 2017 — TVA Medical Inc. announced that its everlinQ 4 endoAVF System has received CE Mark in the European ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
October 2, 2017 — Reflow Medical Inc. announced that the company has received 510(k) clearance from the U.S. Food and ...

October 2, 2017 — Here is the list of the most popular articles and videos on the Diagnostic and Interventional ...
September 29, 2017 — TherOx Inc. announced that the U.S. Food and Drug Administration (FDA) has accepted the premarket ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
September 29, 2017 — Avinger Inc. recently announced Conformité Européenne (CE) Marking approval for treating in-stent ...
Farouc Jaffer, M.D., Ph.D., director of coronary interventions at Massachusetts General Hospital, discusses the newest ...
September 28, 2017 — Abiomed Inc. recently received U.S. Food and Drug Administration (FDA) pre-market approval (PMA) ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
September 27, 2017 — Toshiba Medical announced it will highlight several of its latest vascular and interventional ...
September 27, 2017 — Spectranetics is recalling its Bridge Occlusion Balloon Catheter due to the possibility of a ...
September 26, 2017 — The one-year results of the SENTRY clinical trial were presented by principal investigator Michael ...


 October 17, 2017
October 17, 2017