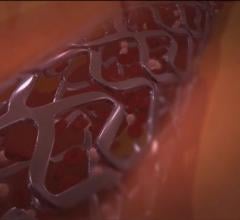
November 3, 2017 — Biotronik's Orsiro drug-eluting stent (DES) demonstrated high long-term safety and clinical ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).

November 3, 2017 — Here is the list of the most popular articles and videos on the Diagnostic and Interventional ...
November 3, 2017 — Acist Medical Systems Inc., a Bracco Group Company, announced the global launch of its Acist RXi Mini ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...

November 2, 2017 – Five-year results from the PREVAIL Trial comparing left atrial appendage closure (LAAC) with the ...

November 2, 2017 — The treatment of in-stent restenosis (ISR) remains challenging in clinical practice. The DARE (Drug ...
November 2, 2017 — Crossing the occlusion with a guidewire is often the most challenging part of chronic total occlusion ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
November 1, 2017 — OrbusNeich reported results from the REDUCE trial in the Late-Breaking Clinical Trial session at the ...
November 1, 2017 — The double kissing (DK) crush two-stent technique was associated with a lower rate of target lesion ...
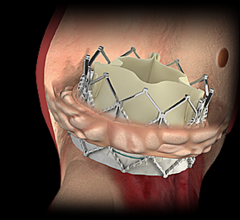
November 1, 2017 — Analysis of the PARTNER 2A trial and the SAPIEN-3 Intermediate Risk registry found transcatheter ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
November 1, 2017 — Results from the prospective, randomized, multicenter CULPRIT-SHOCK trial found an initial strategy ...
October 31, 2017 — The VAMPIRE 3 study evaluated if the selective use of distal filter protection might decrease the ...
October 31, 2017 — Edwards Lifesciences Corp. announced new data demonstrating substantial economic advantages of the ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
October 31, 2017 – Thirty-day results from ABSORB IV, the largest randomized everolimus-eluting bioresorbable vascular ...
October 31, 2017 — The U.S. Food and Drug Administration (FDA) issued a warning to healthcare providers that interim ...
October 30, 2017 — New study results from the EXCEL trial comparing the quality of life (QoL) of patients with left main ...


 November 03, 2017
November 03, 2017