Ted Feldman, M.D., MSCAI FACC FESC, director of the cardiac cath lab, Evanston Hospital, is the principal investigator ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
Vivek Reddy, M.D., director of cardiac arrhythmia services and professor of medicine, cardiology, Mount Sinai Hospital ...
November 13, 2017 — The Society for Cardiovascular Angiography and Interventions recently announced the launch of ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
November 10, 2017 — Cordis, a Cardinal Health company, recently unveiled a comprehensive interventional cardiology portf ...
November 10, 2017 — To address the specific needs of medical imaging clinical engineering departments nationwide ...
November 10, 2017 — GE Healthcare and Dutch-based cardiovascular imaging software provider Medis announced at the 2017 ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
A discussion with Peter L. Duffy, M.D., MMM, FACC, FSCAI CPE, interventional cardiologist and former director of quality ...
November 9, 2017 — TherOx Inc. announced that results of its IC-HOT (Evaluation of Intracoronary Hyperoxemic Oxygen ...
November 9, 2017 — In the Society for Cardiovascular Angiography and Interventions’ (SCAI) comment letter to the Centers ...
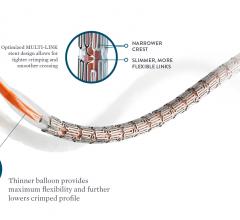
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
November 9, 2017 — 4C Medical Technologies Inc. announced that its medical device for mitral regurgitation (MR) was ...
November 9, 2017 — Abbott received European CE mark for Xience Sierra, the newest generation of the company's Xience ...
A discussion with William W. O’Neill, M.D., medical director, Center for Structural Heart Disease, Henry Ford Hospital ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
November 8, 2017 — Physicians at UnityPoint Health Methodist, Peoria, Ill., are now offering fast, safe and accurate int ...
John Rhodes, M.D., co-director of the adult congenital heart program, Medical University of South Carolina, is the ...
Juan Granada, M.D., Cardiovascular Research Foundation president and chief executive officer, shares his insights on ...


 November 15, 2017
November 15, 2017