The rapid pace of change continues to be a hallmark in cardiovascular medicine and many see that pace accelerating. In ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
January 30, 2018 — The Society for Cardiovascular Angiography and Interventions (SCAI) highlighted the efforts of its ...
January 26, 2018 – Medtronic plc announced the initiation of its investigational device exemption (IDE) study for the ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
January 25, 2018 – Penumbra Inc. today announced results of the company-sponsored PROMISE Study, demonstrating real ...
January 25, 2018 – Advances in brain imaging can identify a greater number of stroke patients who can receive therapy ...
January 25, 2018 – The Intersocietal Accreditation Commission (IAC) announced the release of its Cardiovascular ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
January 25, 2018 – Elixir Medical Corporation, a leader in the development of breakthrough adaptive remodeling ...
January 24, 2018 — Cardiovascular Systems Inc. recently announced two new partnerships broadening the company’s product ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
January 24, 2018 — Boston Scientific Corp. announced it has closed an investment and entered into an acquisition option ...
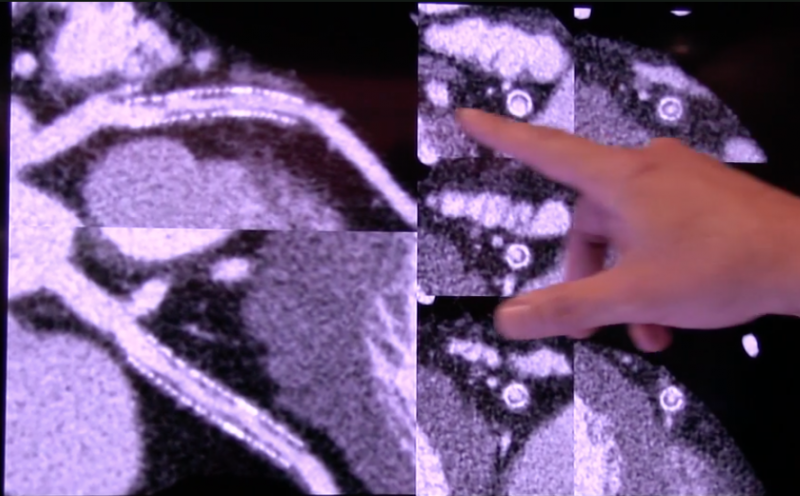
Medical imaging plays a key role in cardiology, and most of the newest radiology technology advances are first unveiled ...

While manual compression remains the gold standard for hemostasis of catheterization vascular access site arteriotomies ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
January 18, 2018 – West Hills Hospital & Medical Center, a full-service acute care facility, announces the first ...
Here is a list of what I think were some of the top interventional technologies discussed at the 2017 Transcatheter ...
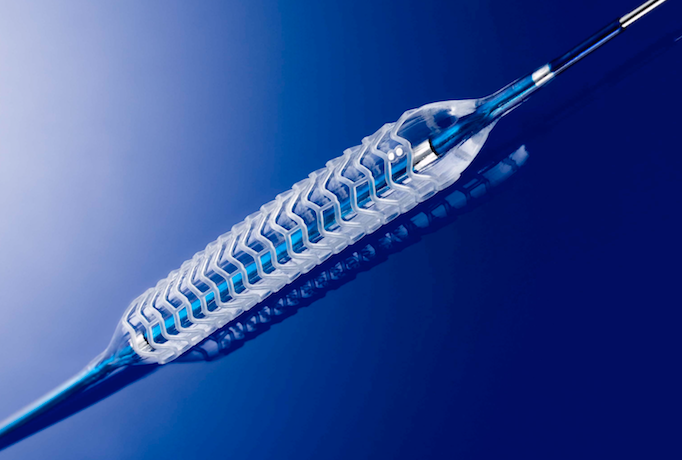
There was no shortage of interest in bioresorbable stent technologies at the many sessions offered on this topic or ...


 January 31, 2018
January 31, 2018













