There are few downsides to using tibial venous access to treat deep vein thrombosis (DVT) with catheter-directed ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
February 15, 2018 — The U.S. Food and Drug Administration (FDA) has expanded the indication for Stryker's Trevo ...
February 15, 2018 — Abiomed Inc. announced that it received an expanded U.S. Food and Drug Administration (FDA) Pre ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
February 15, 2018 — The National Institutes of Health (NIH) awarded a $2.2 million research grant to healthcare ...
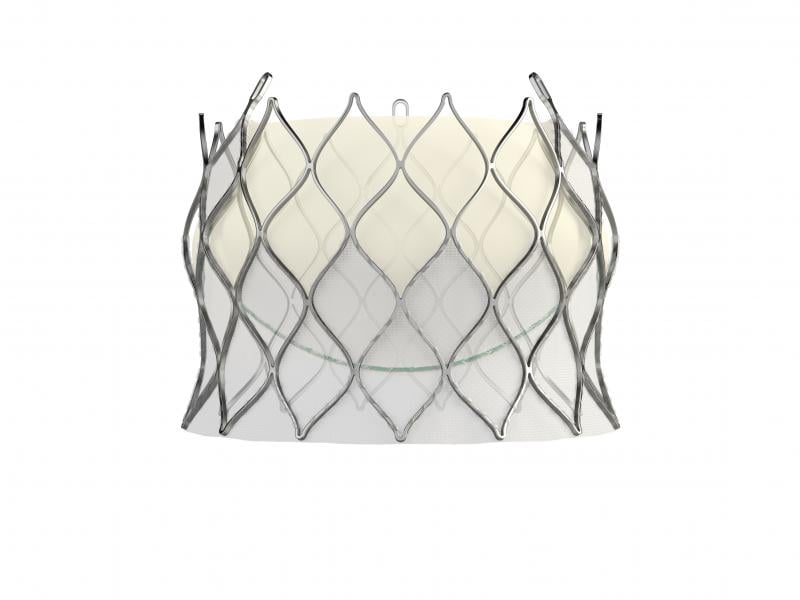
February 15, 2018 — Edwards Lifesciences Corp. has received European CE mark clearance for its self-expanding Centera ...
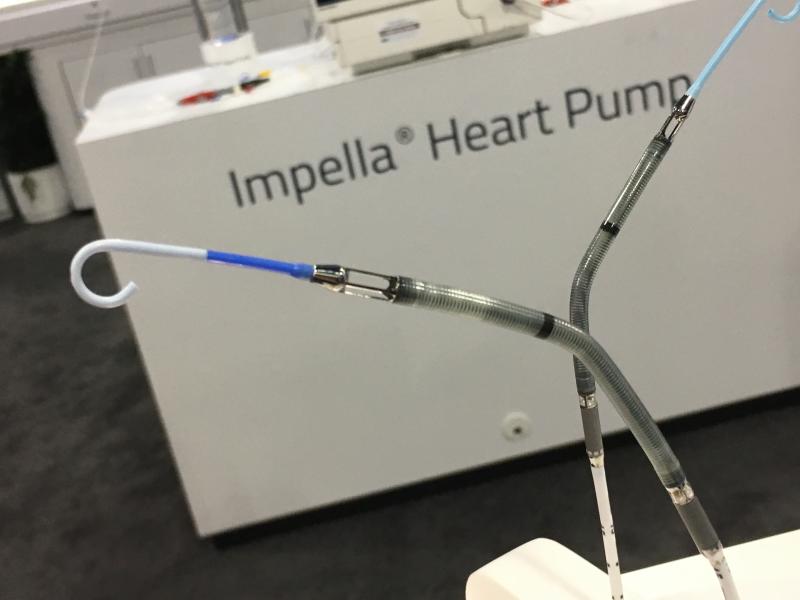
February 14, 2018 — Abiomed Inc. announced it received an expanded U.S. Food and Drug Administration (FDA) pre-market ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
February 13, 2018 — Abbott announced the first patient has been enrolled in a clinical trial evaluating 28 days of dual ...
February 7, 2018 — Medtronic plc recently added to its body of clinical evidence supporting the In.Pact Admiral drug ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
February 7, 2018 — Teleflex Inc. has announced 510(k) clearance by the U.S. Food and Drug Administration (FDA) and U.S ...

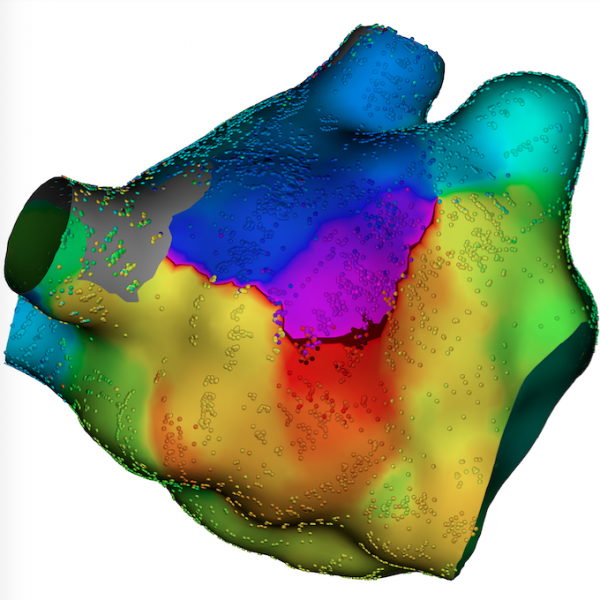
Here is the list of the most popular articles and videos on the Diagnostic and Interventional Cardiology (DAIC) magazine ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
February 1, 2018 – HeartFlow announced that seven new commercial payers issued positive medical policies covering the ...
February 1, 2018 –Stereotaxis, who makes innovative robotic technologies for the treatment of cardiac arrhythmias ...
February 1, 2018 – Thomas Zeller (Bad Krozingen, Germany) presented the 12-month results from Veryan Medical’s MIMICS-2 ...

 February 16, 2018
February 16, 2018