March 25, 2009 - Siemens Healthcare Diagnostics’ C-reactive protein (hsCRP) test is cleared by the FDA for use as ...
Cardiac Diagnostics
This channel includes news, videos, podcasts and other content on new technology innovations for cardiac diagnostic systems and techniques. This includes laboratory testing, blood tests including troponin testing, electrocardiogram (ECG) systems, point of care testing systems, genetic testing, cardiac patient monitoring devices including wearable sensors, and studies showing new ways to diagnose heart diseases.
March 24, 2009 - Ardian Inc said yesterday Medtronic Inc. has led a $47 million financing of the company to ...
March 23, 2009 – The FDA has cleared the Nellcor OxiMax N-600x pulse oximeter with OxiMax SPD alert for adults ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
March 20, 2009 - ARCA biopharma Inc., a biopharmaceutical company developing genetically targeted therapies for ...
March 19, 2009 - The ZOLL Medical Corp. LifeVest Wearable Defibrillator has been prescribed for patients by ...
March 20, 2009 - BRAHMS AG of Hennigsdorf, Germany and Annapolis, MD, recently submitted a 510(k) file to the FDA ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
March 19, 2009 - Sorin Group and Orange Business Services today entered into an agreement to develop and service a ...
March 18, 2009 - GE said yesterday it was awarded two 10-year contracts by the Defense Supply Center Philadelphia ...
March 18, 2009 - Vicor Technologies Inc. has entered into a collaborative research agreement with the University ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The newer ECG devices we have now are so much less cumbersome. It’s like wearing a Band-Aid versus carrying a bulky device,” said the Greenwood, Mississippi internist. “My patients prefer the more comfortable, wire-free form factor, and the quality is as good as, or better, than the Holter,” continued Boler. “Plus, my patient compliance has increased. With the Holter, the leads sometimes come off. The patient may think the device isn’t working, so they take it off and we have to restart the process.”
March 17, 2009 - eCardio Diagnostics has launched an Academic Medicine initiative to provide a tailored offering ...
March 16, 2009 - St. Jude Medical Inc. today announced FDA clearance of the newest version of the Merlin.net ...
Patients with chronic conditions such as congestive heart failure use Honeywell HomMed’s Genesis DM telehealth ...
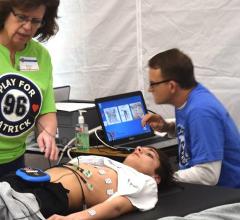
Sudden cardiac death (SCD) is the leading medical cause of death in young athletes and its impact is consistent worldwide. Most professional athletes in the United States are required to take part in comprehensive cardiovascular screening programs to identify often-asymptomatic congenital or inherited heart disorders, and other cardiac risk factors. There remains a debate however, whether to mandate ECGs as part of pre-participation screening programs for student athletes at the collegiate and high school levels or even at younger ages.
March 13, 2009 - Medical testing meets medical texting with the FDA’s clearance of GE Healthcare’s latest ECG ...
March 11, 2009 - The VAP Cholesterol Test from Atherotech Inc. is now available with apolipoprotein A (apoAI) ...
March 11, 2009 – The use of common screening tests, such as the ankle brachial index (ABI), along with ...

 March 25, 2009
March 25, 2009