August 28, 2008 - Just ahead of the expected release of clinical practice guidelines on Familial ...
Blood Testing
This channel includes news and new technology innovations for blood testing including platelet function monitors.
August 27, 2008 - XDx Inc. said today it received market clearance from the FDA for AlloMap Molecular Expression Testing ...
Accumetrics develops and manufactures the VerifyNow System, a comprehensive system for the assessment of platelet ...
August 1, 2008 - Osmetech said this week at the American Association for Clinical Chemistry (AACC) 2008 Conference ...
July 25, 2008 - The FDA granted 510(k) approval in June for numerous diagnostic and invasive cardiac devices, including ...
July 22, 2008 - Osmetech today said it received FDA 510(k) clearance for its eSensor Warfarin Sensitivity Test to ...
July 22, 2008 - CyGene Laboratories Inc. this week is introducing StrokeScan, a genetic screening test aimed at ...
July 7, 2008 - The American Academy of Pediatrics (AAP) today issued new cholesterol screening and treatment ...
July 1, 2008 - The FDA is asking healthcare professionals and facilities check all drug and device storage areas ...
IntelliDOT Corp. received FDA 510(k) clearance for the IntelliDOT Blood Product Administration (IntelliDOT BPA) for ...
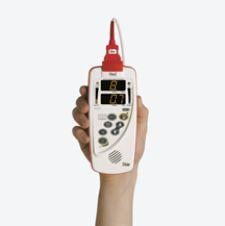
New technology has helped miniaturized bar-code drug administration and patient monitoring systems, eliminating ...
May 21, 2008 - LUMEDX Corp., a provider of cardiovascular imaging and information systems (CVIS), introduced software ...
Masimo received FDA clearance in May 2008 for its noninvasive and continuous total hemoglobin monitoring ...
The FDA cleared for marketing a new automated version of diaDexus’ proprietary PLAC Test, an automated ...
April 28, 2008 - Researchers at the University of Warwick have found a that the protein E-selectin is a marker ...

 August 27, 2008
August 27, 2008

