February 23, 2015 — Medtronic announced that the U.S. Centers for Medicare and Medicaid Services (CMS) has approved a ...
Balloon Catheter
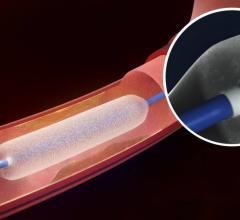
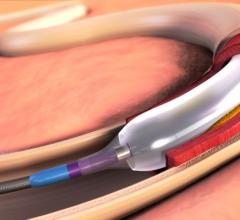
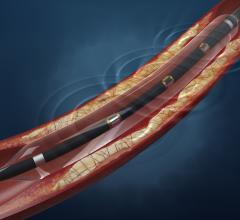
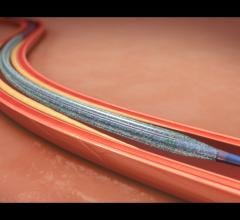
This channel includes news and new technology innovations for angioplasty balloon catheters (PTA). These are used in arteries with atherosclerotic lesions to compress the plaque expand the artery lumen to reopen occluded or heavily stenosed atherosclerotic lesions. Balloons are often used in combination with a stent to prop the treated vessel segment open. In addition to plain old balloon angioplasty (POBA), this section includes news about drug-coated balloon (DCB), valvuloplasty balloons and specialty cutting balloon.
February 23, 2015 — C. R. Bard Inc. announced that the U.S. Centers for Medicare and Medicaid Services (CMS) has ...
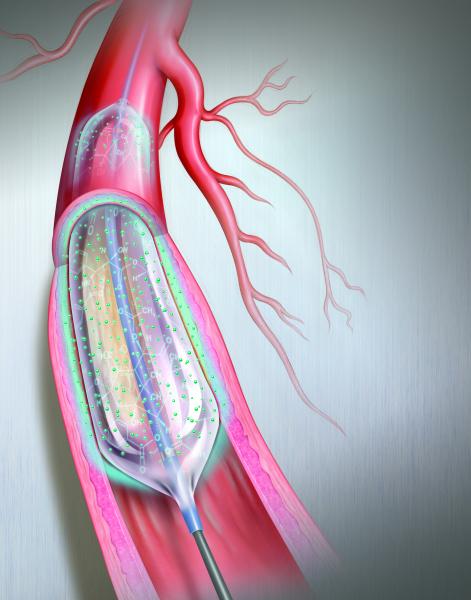
Drug-eluting balloons (DEBs), also referred to as drug-coated balloons (DCBs), have been one of the long-awaited new ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
February 13, 2015 — Boston Scientific and C. R. Bard announced a deal where Boston Scientific will distribute the ...
February 6, 2015 — United States hospitals began using a new medical device from Medtronic plc called the In.Pact ...
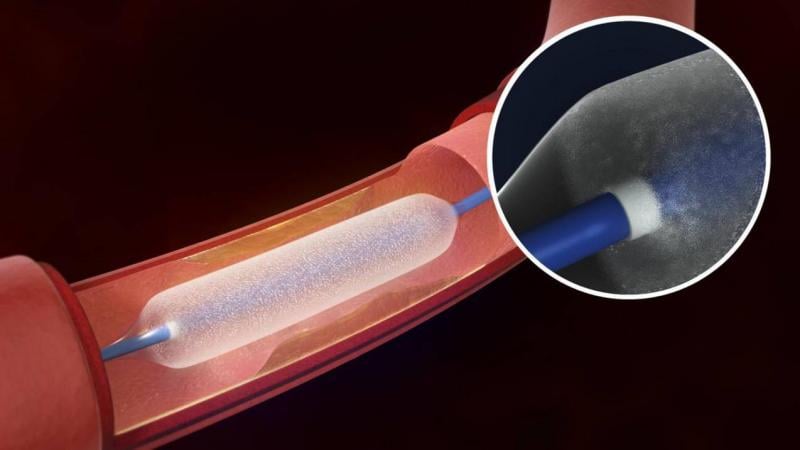
January 30, 2015 — ECRI Institute has created a report that offers an overview of drug-eluting balloon (DEB) technology ...
January 28, 2015 — Maquet Medical Systems USA announced an agreement to serve as the exclusive U.S. distributor of ...
January 13, 2015 — Shockwave Medical announced CE Mark regulatory approval for the company’s Lithoplasty balloon ...
January 9, 2015 — Covidien announced it has received CE Mark approval for its Stellarex drug-coated angioplasty balloon ...
December 29, 2014 — The results of a study published online and in Circulation indicate that a medical device from ...
December 26, 2014 — The results of a landmark study published this month in Circulation indicate that a device from ...
December 19, 2014 — Cardionovum GmbH announced that it has initiated a 150-patient clinical study of its novel paclitaxe ...
December 4, 2014 — Covidien announced that it received U.S. Food and Drug Administration 510(k) clearance for its Fortre ...
Nov. 14, 2014 — Covidien releases 12-month results of the DEFINITIVE AR study, the first randomized study designed to ...

 February 23, 2015
February 23, 2015