October 9, 2015 — Cardinal Health is launching its expanded portfolio in the cardiovascular space including the latest ...
Balloon Catheter
This channel includes news and new technology innovations for angioplasty balloon catheters (PTA). These are used in arteries with atherosclerotic lesions to compress the plaque expand the artery lumen to reopen occluded or heavily stenosed atherosclerotic lesions. Balloons are often used in combination with a stent to prop the treated vessel segment open. In addition to plain old balloon angioplasty (POBA), this section includes news about drug-coated balloon (DCB), valvuloplasty balloons and specialty cutting balloon.
October 1, 2015 — Intact Vascular Inc. announced the U.S. Food and Drug Administration has granted conditional approval ...
August 26, 2014 — The Cardiovascular Research Foundation (CRF) announced the late-breaking trials and first report ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
July 8, 2015 - C.R. Bard Inc. announced the publication of results from the LEVANT 2 study in the June 24, 2015, online ...
June 11, 2015 - C.R. Bard Inc. announced that the U.S. Centers for Medicare and Medicaid Services (CMS) has improved the ...
June 5, 2015 - Medtronic announced the launch of the Euphora semicompliant balloon dilatation catheter in countries that ...
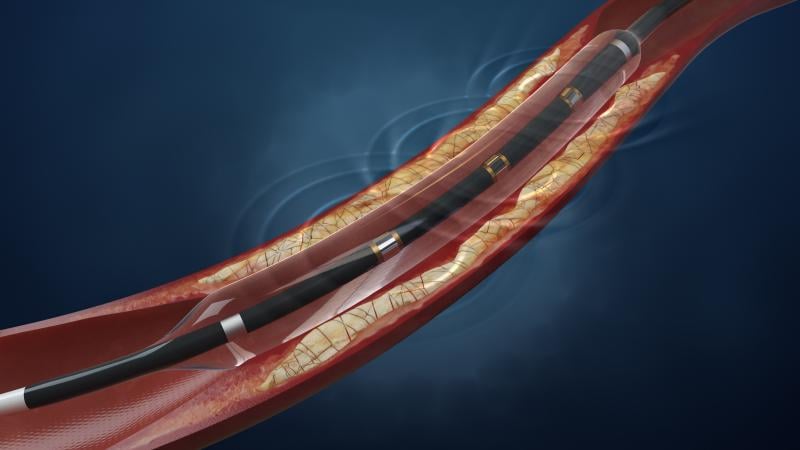
May 27, 2015 — Shockwave Medical announced $40 million in funding, co-led by returning investor Sofinnova Partners and ...
May 22, 2015 — New clinical data from two different studies show the IN.PACT Admiral drug-coated balloon from Medtronic ...
May 8, 2015 — Medtronic plc announced U.S. Food and Drug Administration (FDA) 510(k) clearance and U.S. launch of the ...
May 6, 2015 — The Spectranetics Corporation announced it is accelerating investments in the Stellarex drug-coated ...
In 2015, the FDA approved the first two drug-coated balloons for the U.S. market to treat peripheral artery disease in ...
Hear the latest trends and news on interventional cardiology from the American College of Cardiology (ACC) 2015 meeting ...
March 4, 2015 — Biotronik announced that results of its BIOLUX P-I clinical study have been published in the Journal of ...

March 2, 2015 — Cardinal Health announced plans to acquire Johnson & Johnson’s Cordis business, a leading global ...

 October 09, 2015
October 09, 2015