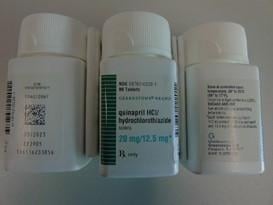
MedWatch, The FDA Safety Information and Adverse Event Reporting Program, announced that Pfizer is recalling Accuretic (quinapril hydrochloride/hydrochlorothiazide) tablets distributed by Pfizer as well as two authorized generics distributed by Greenstone (quinapril HCL and hydrochlorothiazide and quinapril HCl/hydrochlorothiazide) due to the presence of a nitrosamine, N-nitroso-quinapril, above the Acceptable Daily Intake level.
Here is what you and your colleagues found to be most interesting in the field of cardiology during the month of March. This data was drawn from dicardiology.com’s 163,000 viewers over the course of the month.
1. FDA Recalls Quinapril Hydrochloride and Hydrochlorothiazide Tablets by Pfizer
2. David Bennett, Pig Heart Transplant Patient, Dies
3. ACC 2022 Late-breaking Clinical Trials Announced
4. Experts Perform Pathogen Surveillance for Unprecedented Pig-to-Human Heart Transplant
5. HLT, Inc. Gains FDA Approval for Two TAVR Clinical Studies
7. An Accurate and Low-Risk Diagnostic Test for Coronary Artery Disease
8. Late-Breaking Study Results Reinforce Positive Real-World Outcomes with the WATCHMAN FLX LAAC Device
9. Novel Technology Could Prevent Repeat Operations to Replace a Faulty Bioprosthetic Heart Valve
10. CIRCA Scientific Receives FDA Clearance for New Esophageal Temperature Probe


 July 31, 2024
July 31, 2024 









