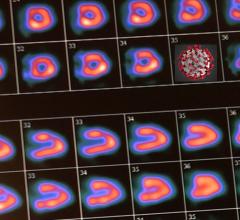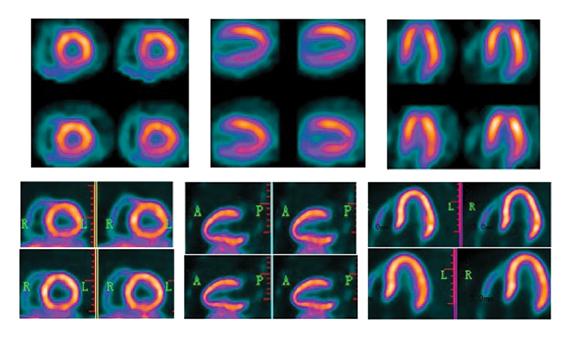
BFPET demonstrates higher resolution in the evaluation of myocardial perfusion in this series of images contrasting the results of a PET scan done with FluoroPharma's fluorine-18 based agent and a SPECT study done with sestamibi. Images were captured during an investigator-initiated study of BFPET conducted at 301 Hospital in Beijing, China.
Just when positron emission tomography (PET) appears to be eclipsing single photon emission computed tomography (SPECT) for cardiac imaging, new advances make SPECT more attractive. Both modalities also have suffered setbacks with radiopharmaceutical supply problems in recent years and both modalities have their pros and cons. Looking toward the future, the question of which modality will dominate remains unanswered. PET shows major promise with exciting new tracers, while new SPECT scanner technology introduced at the Society of of Nuclear Medicine and Molecular Imaging (SNMMI) 2013 meeting may herald a rebirth for SPECT with previously unseen image quality enhancements.
Watch the 2017 VIDEO “Trends in Nuclear Cardiology Imaging. David Wolinsky, M.D., director of nuclear cardiology at Cleveland Clinic Florida and past-president of the American Society of Nuclear Cardiology (ASNC), discusses advancements in nuclear imaging and some of the issues facing the subspecialty.
At SNMMI 2013, Siemens introduced innovative new technologies for both PET and SPECT. The vendor introduced what it calls xSPECT on its 510(k)-pending Symbia Intevo SPECT/CT. In traditional SPECT/CT imaging, the SPECT scanner image has always been reconstructed at a low-resolution matrix — much lower than the CT portion of the exam. As a result, the CT resolution must be downgraded dramatically to the level of SPECT to enable mechanical fusion of the two modalities. xSPECT combines the images into a new, completely integrated single dataset for vastly higher resolution and quantitative images.
Aimed at the oncology market to start, an example displayed at SNMMI showed a traditional SPECT/CT spinal image with hot spots, but the low resolution makes it unclear if the areas of enhancement are tumors or inflamation. The combined xSPECT image greatly enhanced resolution to show the hot spots were actually inflammation caused by clearly defined spinal compression fractures. The clear, fused image also eliminates the alignment artifacts common with hybrid imaging, where the SPECT and CT images do not exactly match anatomical boundaries. The vendor says centers using the system in trials are reading the xSPECT image rather than going back and forth between the usual collage of three images showing the SPECT, CT and combined overlay image.
SPECT has traditionally been dubbed “unclear” instead of “nuclear” imaging due to its low image quality, said Mike Rittman, senior manager, product marketing, Siemens molecular imaging. He explained xSPECT offers a way to greatly clarify the image with CT-like quality. Instead of an increamental technology advance, Rittman said this is a major step forward for SPECT, involving 10 years of development and 32 pounds worth of paperwork submitted to the U.S. Food and Drug Adminitsration (FDA) for the approval process.
Siemens also introduced the FDA-cleared Biograph mCT Flow PET/CT system, which is the first to offer continous motion scans, rather than step-and-shoot acquistions. It uses a magnetically levitated bed that moves the patient smoothly through the system’s gantry. A standard PET/CT scan can last 25 to 30 minutes, with the patient frequently falling asleep while the system acquires a bed position. When the bed shifts to the next scanning position, the patient is often startled, resulting in an image that is marred by motion artifacts. The patient also can feel anxiety during the lengthy scanning process, perceiving no progress until the bed shifts to a new scanning position. Siemens had to develop new ways to process the continuous flow of massive amounts of data from the scanner.
The mCT Flow also removes the limits of the standard 16 cm scan area, which often requires image overlap for stitching and scanning additional areas that are not required. Rittman said this can lower radiation doses considerably, since the scan length can be set by the operator instead of using 16 cm increments.
PET vs. SPECT Technology
The future success of PET may be grounded in its inherently better image resolution. In cardiac scanning, it has generally been reported that PET offers a resolution of 5 to 7 mm, compared with a cardiac SPECT resolution of 12 to 15 mm. Better performance has allowed data to emerge suggesting that as many as one in 10 scans interpreted as normal on SPECT would have been abnormal if done on PET due to the presence of unseen microvascular, triple-vessel disease. PET’s superior diagnostic capability is achieved partly through advances in hardware, particularly quantification, which leverages numerical precision to identify global perfusion defects in the heart that otherwise might be hidden from qualitative SPECT scans.
PET may have an ace in the hole in its ability to quantify results. Quantification is now being built into PET/CTs sold into mainstream medical practices. SPECT scanners lack the resolution of PET/CTs and SPECT vendors have yet to commercialize systems capable of quantification.
A big difference between the two technologies is the half-life of the isotope that each radiopharmaceutical tracer uses. SPECT tracers have a relatively long half-life (technetium-99m has a half-life of six hours), whereas rubidium-82 is only 75 seconds. This short half-life is a limitation of the current front-line
cardiac PET radiotracer, which does not leave much room for error when imaging and presents the inability to do exercise stress testing.
New iterative reconstruction (IR) software such as UltaSPECT is improving SPECT image quality by boosting the signal-to-noise ratio. Just as in CT scans, IR can also help reduce dose by enhancing lower-quality scans.
Advances in Radiotracers
The next generation of PET imaging agents might herald an age when PET will eclipse SPECT as the “go to” modality for molecular imaging, according to Thijs Spoor, chairman, CEO and president of FluoroPharma, a company developing new PET radiotracers. He explained PET will do so by enabling personalized medicine through precision diagnostics, the ability to be delivered cost-effectively in a manner with less radiation to patients, by leveraging hardware advances already being commercialized. PET also is taking advantage of the extra throughput capacity present in the U.S. installed base of PET/CT scanners.
Fluorodeoxyglucose (FDG) created the oncology business for PET, and its growth was explosive, Spoor said. But while FDG is clinically very good, he says it is not perfect. It does not work on all cancers and only provides a look at the basic metabolism. This is opening the door for the next generation of oncology agents that are specific to cancers not well-characterized by FDG.
“While there is buzz around new PET agents in neurology for detecting beta amyloid plaques associated with Alzheimer’s disease, it is cardiology that presents some of the most exciting opportunities for PET,” Spoor said. “New cardiac PET agents promise robust, reproducible and dependable results in everyday cardiology practices and the need for these agents is mind-boggling.”
Cardiovascular disease is the single largest cause of global mortality, causing more than 17 million deaths each year, exacting an annual financial cost around the globe of a staggering $863 billion. In the United States alone, 729,000 people die from cardiovascular disease annually. Annual U.S. health costs are estimated to be $280 billion, rising to $800 billion by 2030.
New PET Radiotracers
Rubidium-82 technology has established benchmarks in the assessment of myocardial perfusion against which the next generation of cardiac agents can be measured for sensitivity and specificity. It also is producing data that should demonstrate the cost-effectiveness of cardiac PET over SPECT, and has pioneered the way for cardiac PET through the issuance of CPT codes and reimbursement policy by third-party payers for PET myocardial perfusion. But rubidium-82 has drawbacks that may limit its sustained clinical adoption as seen in recent publications. These limitations include supply chain issues, economics, its short half-life and potential safety issues as presented in the black box warning in the package insert. These issues should be resolved by the coming wave of new fluorinated cardiac PET radiopharmaceuticals.
Fluorinated positron emitters that compose the next generation of PET radiopharmaceuticals offer benefits over rubidium, including a longer half life of about 109 minutes. They also offer some clinical possibilities in the diagnosis and monitoring of cardiovascular disease beyond myocardial perfusion. Expected advantages of these new agents over SPECT radiopharmaceuticals are improved diagnostic accuracy, image clarity and lower radiation exposure to patients.
Lantheus Medical Imaging’s Flurpiridaz F-18 myocardial perfusion imaging agent is currently being evaluated in Phase III clinical studies. The agent can be used with exercise stress (PET is currently limited by its short half-life agent to pharmaceutically induced stress), and has a high extraction rate of up to 90 percent by the mycardium, which can help reduce the amount of agent and the radiation dose to the patient. Also, F-18 tracers are already being produced and have an established distribution network.
FluoroPharma’s BFPET, a fluorinated myocardial perfusion agent, exemplifies how the next generation of cardiac PET technology might boost diagnostic performance. In a study patient, BFPET indicated that the patient did not have a perfusion defect, as was suspected on the basis of SPECT, but rather suffered from apical thinning. Cardiovascular disease was confirmed through CT angiography — as was the absence of a perfusion defect.
BFPET has the potential to establish a new standard for measuring cardiovascular blood flow. The agent is being developed for use in combination with stress testing in patients with presumptive chronic coronary artery disease (CAD), as a replacement for SPECT in institutions with PET capability.
Healthcare reform emphasizes cost-effective care, so it is increasingly important to minimize the number of tests performed. The significance of getting it right the first time rises when considering the danger of cumulative radiation exposure to the patient, where the best diagnostic test done first might eliminate the need for subsequent invasive, expensive or unnecessary tests.
Several next-generation cardiac PET agents are designed to address unmet clinical needs. Whereas BFPET promises increased diagnostic accuracy compared with thallium and technetium-based SPECT agents, as well as advantages that go beyond the PET agent rubidium-82, CardioPET transcends even that, Spoor said. This agent, another in FluoroPharma’s pipeline, accesses a novel metabolic pathway, one that involves fatty acids, the primary source of energy for the cardiac muscle.
“Our studies indicate that imaging with CardioPET can potentially be used to accurately gauge fatty acid uptake by the myocardium. This uptake can be visualized and quantified using PET scanners that are becoming more available at price points within the grasp of routine practitioners,“ Spoor explained.
Preliminary data indicates that the agent is especially suited for the diagnosis of acute coronary syndrome and chronic coronary artery disease in patients who cannot undergo stress testing.
By obtaining list-mode data on modern scanners, CardioPET may allow measurements of perfusion during the first five minutes of administration while scans done 40 to 60 minutes later may indicate tissue viability. These two clinical capabilities suggest the potential utility of CardioPET as a way to assess patients after they have complained of chest pain in the emergency department.
PET may offer an imaging option to detect heart failure and the risk of sudden cardiac death by imaging the sympathetic nervous system using the cardiac neuronal agent LMI 1195. Lantheus has this agent in a Phase I trial.
Research is ongoing for use of FDG F-18 agents to image cardiac sarcoid to identify patients at risk for sudden death. “We see huge promise to inject these patients with FDG to check for sarcoid, and if they have it, we can get them treated with an ICD [implantable cardioverter defibrilator] before they end up in a fatal sitaution,” said April Mann, CNMT, NCT, FSNMMI-TS, manager of noninvasive cardiology, Hartford Hospital, during a presentation at SNMMI 2013.
Another exciting area of research mentioned by several SNMMI presenters is the use of coronary flow reserve as a way to quantify the severity of coronary lesions, similar to fractional flow reserve (FFR) measurements made invasively in the cath lab. F-18 agents may offer the best imaging agent for this type of quantification due to its high myocardial uptake and eliminate the need for diagnostic catheterizations.
New SPECT Radiotracers
On the SPECT front, FDA in March approved a new indication for GE Healthcare’s agent AdreView (iobenguane I-123) as the first agent to link nerve function in the heart to a patient’s mortality risk. AdreView is approved for the scintigraphic assessment of myocardial sympathetic innervation (cardiac nerve activity) to assist in the evaluation of patients with New York Heart Association (NYHA) Class II or Class III heart failure and left ventricular ejection fraction (LVEF) ? 35 percent. Increased myocardial sympathetic activity is a prominent feature of heart failure and is often associated with decline in LV function, worsening heart failure symptoms and sudden cardiac death. This increase leads to a depletion of norepinephrine (NE) storage and uptake. AdreView provides a means for assessing the neuronal capacity for uptake of NE.
In January, Lantheus began shipments of its new LEU TechneLite generator, which is the first technetium-99m (Tc-99m) generator in the United States that contains molybdenum-99 (Mo-99) produced from at least 95 percent low-enriched uranium (LEU). Through its supply chain diversification strategy, Lantheus plans to eventually eliminate the use of highly enriched uranium (HEU)-sourced Mo-99 from its supply chain. This is also in part due to a federal effort to eventually reduce the availability of HEU to aid interventional efforts to stop nuclear weapons proliferation.
Imaging Vulnerable Plaques
VasoPET, a next-generation positron biotracer being developed by FluoroPharma, is designed to address the unmet clinical need of identifying vulnerable plaques. These plaques are likely to rupture and cause a stroke or heart attack. Preclinical tests show that this fluorine-bearing radiopharmaceutical is taken up by inflammatory cells not found in stable plaque.
This agent is still in preclinical development, so it remains a speculative technology. The prospect is extraordinarily appealing for an agent that might be used in high-risk patients, particularly those being readied for cardiac catheterization.
“This technology could help the interventional cardiologist identify blood vessel segments that have vulnerable plaques to avoid them and the possibility of creating emboli in the vessel. In the future, this imaging might be used to preventively identify and treat these lesions. An imaging agent such as VasoPET would also be helpful in the development of therapeutics being tested for their ability to dissolve vulnerable plaques,” Spoor said.
Other modalities, including CT and MRI, cannot readily differentiate inflamed from stable plaques.
The challenge facing all these agents is whether they can deliver clinically significant results in the hands of routine practitioners and not just once in a while. Their results must be consistent and reproducible, rendering diagnostic and prognostic conclusions regardless of practitioner skill.
Serious Issues With Isotope Supply
Technetium, which fuels the majority of SPECT procedures in cardiology, has suffered several disruptions in supply over the past decade. A reactor at Chalk River Laboratories in Ontario, Canada, normally supplies about half of the world’s molybdenum, which serves as a generator of technetium. The vast majority of the technetium generators are used in the United States. Canada plans to permanently shut down the reactor in 2016 due to its age and safety concerns. Thus far, no other North American sources of molybdenum have surfaced to make up for this deficit.
In the past, supply disruptions have seriously impacted the nuclear medicine community. When a radioactive leak sidelined the reactor in 2009, extreme shortages of technetium resulted over the 15 months the reactor was out of service. Because molybdenum has a half-life of 66 hours, shipments from overseas reactors to the United States caused significant logistical headaches.
Some physicians turned to the PET tracer rubidium-82 to fill in until news broke last year that improper daily monitoring of the generator was resulting in strontium breakthroughs that left radioactive material in the patient long after a perfusion exam. While crossing the border between the United States and Canada in July 2011, two patients who had recently undergone rubidium-82 PET scans set off radiation alarms. It was learned that these patients had been inadvertently exposed to large doses of strontium contaminant, the parent isotope used in the generation of rubidium. Rubidium-82 was voluntarily taken off the market by Bracco pending review by the FDA and did not return to market until nine months later, after the FDA traced the fault to improper handling of the generator, not its manufacture. The incident has had lasting effects, however, on the molecular imaging community. Today, those using rubidium-82 must undergo special training and are subject to daily reporting requirements.
Currently, the U.S. Department of Energy (DOE) is the only domestic supplier of the isotope strontium-82 used in rubidium-82 generators. Positron Corp. is working with the FDA for certification so it can begin its own production of strontium-82, through its subsidiary Manhattan Isotope Technology, to provide a second source for the PET cardiac perfusion isotope.
Fluorinated positron emitters could be produced with relative ease in the United States, and the methodology for handling the fluorine-18 radioisotope has been vetted by decades of use and millions of procedures involving FDG without mishap, said Spoor. Fluorine-18 has a lower dose profile than SPECT radioisotopes, reducing the overall radiation burden placed on the patient. Dual isotope SPECT scans may expose the patient to between 25 and 30 mSv of radiation. SPECT rest-stress with technetium exposes the patient to between 8 and 10 mSv. In contrast, exposure from fluorine-18 scans range from 4 to 8 mSv, Spoor explained.
To the benefit of the molecular imaging community and the future growth of this modality, the widespread use of FDG with its utility in oncology led to a rapid expansion of the installed base of PET/CT scanners and establishment of an extensive and nationwide FDG distribution network. About 1,800 PET/CTs are operating in the United States and are served by about 100 commercial FDG production sites. Many of these scanners are running at only about half capacity. Consequently, early adopters of the next generation of PET radiopharmaceuticals will have ready access to the needed hardware to perform scans.
“It makes sense when performing medical imaging procedures to go with the modality that delivers the best image quality at the lowest dose, one that renders reproducible results regardless of the skill of the practitioner and is not vulnerable to supply-line disruptions. The case is that much stronger when this modality gains access to specialized radiopharmaceuticals that promise cost-effective and clinically significant testing that will expand the capability of routine healthcare practitioners in neurology, oncology and cardiology,” Spoor said.
Related Nuclear Cardiology Content
Recent Advances in Cardiac Nuclear Imaging Technology (Sept. 2017)
Watch the VIDEO “Trends in Nuclear Cardiology Imaging.
Read the article “PET vs. SPECT — Will PET Dominate Over the Next Decade?”
Read the article “SPECT vs. PET, Which is Best?”
Find out more about the current state of PET imaging technology with the article "PET Imaging 101."
Advances in Cardiac Nuclear Imaging
Moving Awareness to Action in Nuclear Medicine Dose
VIDEO: PET vs. SPECT in Nuclear Cardiology and Recent Advances in Technology - Prem Soman, M.D., director of nuclear cardiology at the Heart and Vascular Institute, University of Pittsburgh, and president-elect of the American Society of Nuclear Cardiology (ASNC), explained advances in PET and SPECT imaging and the learning curve involved in reading scans from the new CZT SPECT cameras.
Editor’s note: Thijs Spoor, FluoroPharma chairman, CEO and president, contributed to this article. He has more than 15 years medical industry experience. He is a former securities analyst at J.P. Morgan and Credit Suisse, covering the medical device industry. Before joining FluoroPharma, he led the nuclear cardiology portfolio and PET new product opportunity portfolio at Amersham/GE Healthcare.


 November 12, 2025
November 12, 2025