Getty Images
The European interventional cardiology device market is one of the larger markets in the global space. The market is driven by the regions aging population and the health care systems demands for various medical devices. However, like the rest of the globe, Europe is seeing a rapidly aging population who are requiring more cardiac care in addition to a decline in overall cardiovascular health. The rise in demand comes as the regions market matures with continuing depreciating prices. At the same time, there is a stronger investment in new innovative premium products. The investment is partially driven by the varying national health care reimbursement schedules. As each country has their own health system, the coverage varies from extensive in the Nordic region to a mixed system in Italy. This new investment is expected to mitigate the reduction in Europe’s interventional cardiology market value. It is expected that the COVID-19 pandemic has not led to changes to these trends; but rather, it has shown the need to continue to invest.
Demographic Changes
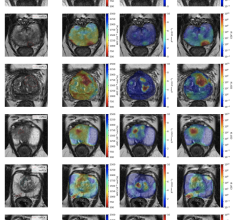
According to the United Nations (UN), the process of population aging is most advanced in Europe and in North America, where approximately 25% of the population was aged 60 or over in 2017. By 2050, older persons are expected to account for 35% of the population in Europe and by 2070, this number is expected to exceed 50%. Globally, Italy, Germany, Portugal and Finland are all ranked in the top five oldest populations. As the number of people over the age of 60 in a population increases, the risk of cardiac events also rises. The shifting demographics is reflected in the number of expected angiography and angioplasties/PCIs over the period the report. Both procedures are seeing a minor increase, with the use of atherectomies, optical coherence tomography (OCT) and intravascular ultrasound (IVUS) replacing the former two in many cases. In addition to aging, the shift towards a more sedentary lifestyle has led to a rise in cardiovascular disease across Europe. It is estimated that a little under half of all deaths in Europe are caused by cardiovascular disease. The increase of doctors performing tests through angiography and other diagnostic procedures is a testament to how prevalent cardiovascular disease is becoming. While there is some gained attention to reducing the risk of cardiovascular disease, it is expected that the prevalence of the disease will remain a key demographic shift. It is expected that in response to the disease rate, healthcare practitioners will increase the amount of diagnostic testing in order to keep the European population healthy.
Shifting Market Dynamics
The European interventional cardiology market has reached maturity and is now seeing price depreciation leading to market value declines across most of the market segments. The exceptions to this trend include the drug-eluting balloon, coronary atherectomy, OCT and IVUS markets. Due to recent investments these markets have had new products enter the market space, bringing new growth and value to the mature markets. The three segments are seeing declining market value over the reports forecast period; however, an increase in units sold is mitigating the decline. Driving the growth is the fact that there is heavy investment from companies for these premium products. The European market has the willingness to pay for these higher priced devices due to the regional reimbursement policies. Places like Germany are expected to see some of the highest use as they offer higher coverage across the region. As a result of this willingness to pay companies like ShockWave Medical, for its intravascular lithotripsy (IVL) technology, companies are starting to establish themselves in the region. The market penetration of the new technology is still up in the air. While it remains a niche market with less investment compared to IVL, drug-eluting balloons are starting to receive regional approval. In Europe, select drug-eluting balloons are now approved for use giving new value and care options to the balloon catheter market. As they are still new and not yet approved in the United States, they have not caused any market shifts yet, but in a few years the presence is expected to make an impact. As emerging research shows that reductions in procedure complications is being linked to the drug-eluting balloons. Based on the trajectory of drug-eluting stents, this gives the drug-eluting balloon market a space to keep watch on.
Impact of COVID-19 Pandemic
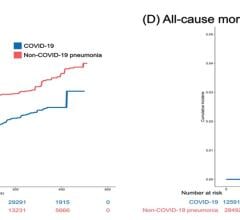
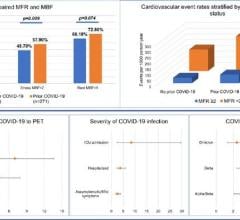
Now entering the second year of recovery from the COVID-19 pandemic, the European market has returned to trends set in 2019, prior to the pandemic. Throughout 2020 the number of procedures in the region declined between nine and thirteen percent. Due to the varying scale of procedure cancelations and lockdown durations each country experienced differing impacts. The United Kingdom and Portugal saw some of the largest declines in 202. While Switzerland and Scandinavia saw a much lower decline. In the United Kingdom a longer lasting impact is the wait list for cardiac procedures, which has now doubled as throughout 2020 cardiac procedures were deemed elective and so were canceled. It is worth noting that the National Healthcare System (NHS), in the United Kingdom was already seeing a trend of long wait times prior to the pandemic. The pandemic only strengthened the trend. However, across the region most restrictions were lifted by mid-2021 as a result of immunizations and maintained lower case numbers. This resulted in a temporary surge in the interventional device market which lingered in 2022 and has now faded in 2023.
Final Thoughts
The European market will see a shift on both the consumer and producer sides of the interventional cardiology market. As the population ages and cardiovascular health declines, it will fuel demand for cardiac procedures. In response the companies will continue to invest in premium new products that allow for increased efficiency in caring for the European population. Given the gained efficiency of these premiums, it is expected that the use of them will significantly increase, as the health care systems deal with the backlog of cardiac cases. All leading the great outcome of a healthier continent.

Emily Gilbert is a research analyst at iData Research. She develops and composes syndicated research projects regarding the medical device industry, publishing the U.S. Interventional Cardiology series.

Kamran Zamanian, PhD, is CEO and founding partner of iData Research. He has spent over 20 years working in the market research industry with a dedication to the study of medical devices used in the health of patients all over the globe.

 March 20, 2024
March 20, 2024