March 9, 2009 – The Cardiology Clinic of San Antonio and Cardiology Consultants of Houston in Texas have chosen Digisonics’ DigiNet Pro System for their cardiovascular image management and reporting needs.
March 10, 2009 - ProSolv CardioVascular, a FUJIFILM company, has released its newest software version Synapse ProSolv cardiovascular 4.0.1, featuring an integrated ECG management capability and integration with the Siemens Sensis Hemodynamic system. Synapse ProSolv cardiovascular 4.0.1 will be one of several new products highlighted at ACC 2009 this month.
The Cardiac Rhythm Center at John Muir Health in the San Francisco Bay area of California performs electrophysiology (EP) procedures on a system that integrates diagnostic and therapeutic devices, workstations, data repositories and reporting functions.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Door-to-balloon time can be used as a quality measure for hospitals in their level of excellence in caring for patients with acute ST-elevation myocardial infarction (STEMI). It can also be used as a conceptual framework for thinking about quality improvement, differences between outcomes-focused and process-focused quality programs.
The Washington Hospital, an acute care facility serving primarily an elderly patient population, performs approximately 4,300 cardiac and vascular procedures annually. Due to a recent expansion, The Washington Hospital’s Heart and Vascular Center needed to upgrade its equipment and its critical information systems.

Congenital heart disease contributes to more than 30 percent of infant mortality in the U.S., so time is essential in diagnosing and treating these conditions in newborns. However, most hospitals do not have pediatric cardiac specialists on staff who can diagnose and suggest courses of treatment, which is why telecardiology has bloomed in this field.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

The dividing line between the jobs performed by cardiac surgeons and interventionalists will become increasing blurred in the near future as transcatheter heart valves and PFO occluders replace open-heart surgery. These nonsurgical structural heart treatment techniques were among some of the most exciting trends at TCT 2008, which was highlighted by nearly 40 sessions on these subjects.

With the healthcare industry striving for greater efficiency at virtually every level, it shouldn’t be a surprise that hospitals and clinics are increasingly turning to manage patients with implantable cardiac devices remotely, including implantable cardioverter-defibrillators (ICDs), cardiac resynchronization therapy-defibrillators (CRT-Ds) and pacemakers.

Management of implanted leads for pacemakers and defibrillators is a growing concern among physicians with more than 2 million Americans relying on a pacemaker or defibrillator to regulate their heart beat and that number growing each year. The lead is the most vulnerable part of an implantable device and the most common component to fail.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
March 9, 2009 - The use of dual-source computed tomography (DSCT) may be useful to noninvasively detect in-stent restenosis, especially in stents with a relatively large diameter, according to a study in this week’s issue of the American Journal of Cardiology (pages 812-817).
March 9, 2009 – Merck & Co. Inc. and Schering-Plough Corp. today said their Boards of Directors unanimously approved a merger agreement under which the companies will combine under the name Merck.
March 9, 2009 - Ziosoft’s new monthly subscription program for its Ziostation software will allow facilities to utilize Ziosoft's thin-client system immediately without a capital investment. In addition to the monthly subscription fee program, the company will also offer flexible purchasing plans tailored to customers' needs.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
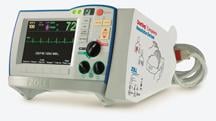
March 9, 2009 - The Ministry of Health for the Province of Quebec has awarded ZOLL Medical Corp. a contract valued in excess of $12 million to equip all ambulances in the Canadian province with the ZOLL E Series defibrillator, representing the first time the Province of Quebec will standardize its entire system to one model of defibrillator.
March 9, 2009 - Cytori Therapeutics Inc. completed enrollment in its APOLLO trial to evaluate the safety and feasibility of adipose tissue-derived stem and regenerative cells, processed with the company’s Celution System, in the treatment of severe heart attacks.
iNtuition is TeraRecon's new, unified workflow platform, which delivers the best possible solution for advanced image management in an enterprise and an efficient way to manage workflow once the CT data leaves the MDCT scanner.


 March 10, 2009
March 10, 2009










![TeraR New_145x145_ITNfastpass[2]](/sites/default/files/styles/content_feed_medium/public/TeraR%20New_145x145_ITNfastpass%5B2%5D.jpg?itok=4Ca-4wty)
