May 10, 2009 - Edwards Lifesciences Corp. Friday said it received clearance from the FDA for the Carpentier-Edwards PERIMOUNT Magna Ease aortic valve, designed for easier implantation in the heart.
May 10, 2009 - Biosite and the FDA notified healthcare professionals of the Class 1 recall of the Biosite brand Triage Cardiac Panel, a test is used by as an aid in the diagnosis of a heart attack (myocardial infarction).
May 10, 2009 - Medtronic Inc. Friday announced the settlement of all royalty disputes with Johnson and Johnson (J&J) that concern Medtronic’s licensed use of the Palmaz, Schatz and Pinchuk patents relating to coronary angioplasty stent design and balloon material patents.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
May 8, 2009 – Siemens Healthcare announces FDA 510(k) clearance for the new Biograph TruePoint 16-slice PET/CT system, which is now commercially available.
May 8, 2009 - Positron Systems Inc. signed a Letter of Intent with Idaho State University (ISU) that will set the stage in a public/private partnership for the production and distribution of molybdenum-99 (99Mo), a widely used isotope in nuclear medicine.
May 8, 2009 - Preliminary data presented yesterday at the 32nd annual SCAI Scientific Sessions show that 82 percent of patients treated with Cook Medical’s Zilver PTX drug-eluting peripheral stent were free from reintervention at two-year follow up.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
May 8, 2009 - ProSolv CardioVascular said Allina Hospitals and Clinics in Minneapolis, Minn., recently selected the Synapse ProSolv cardiovascular solution to become its enterprise cardiovascular system.
May 8, 2009 - The 63rd Vascular Annual Meeting hosted by the Society for Vascular Surgery is expected to draw 1,200 of the world's top vascular surgeons June 11-12 at the Colorado Convention Center in Denver.
May 7, 2009 - The University of Illinois Medical Center, the primary teaching facility for the UIC College of Medicine, is the first such institution to install and the innovative Shape-HF Cardiopulmonary Testing System.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
May 7, 2009 - GE Healthcare and Hansen Medical Inc. today said they have entered into a collaboration to promote the use of GE Healthcare’s imaging technologies and Hansen Medical’s robotic catheter technologies for electrophysiology (EP) procedures.
May 7, 2009 - Medtronic Inc. yesterday said it completed enrollment in its investigational device exemption (IDE) study of the Endurant Stent Graft System, which is designed to enable the nonsurgical repair of aortic aneurysms. Key data from this study, now expected in the second half of 2010, will be used to support the FDA pre-market approval (PMA) submission for U.S. approval of Endurant.
May 7, 2009 - The Society of Cardiovascular Computed Tomography (SCCT) said this week a report, “Guidelines for the Performance of Coronary Computed Tomographic Angiography,” will be printed in the May/June issue of the Journal of Cardiovascular Computed Tomography that establishes a consensus of the minimally required standards for appropriate coronary CTA acquisition, as well as recommendati
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
May 7, 2009 - St. Jude Medical Inc. today started the U.S. launch of its Attune Flexible Adjustable Annuloplasty Ring for the repair of diseased heart valves. The company received FDA clearance and will introduce the product at the annual meeting of the American Association for Thoracic Surgery May 9 in Boston.
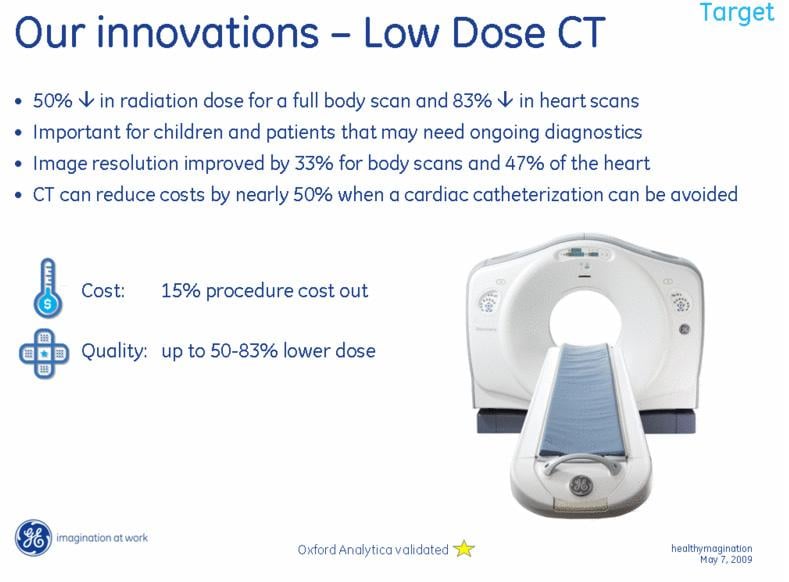
May 7, 2009 - GE announced today that it will spend $3 billion over the next six years on healthcare innovation that will help deliver better care to more people at lower cost. In addition, the company will commit $2 billion of financing and $1 billion in related GE technology and content to drive healthcare information technology and health in rural and underserved areas.
GE Healthcare's CardioICE (intra-cardiac echo) is offered as a feature on the new CardioLab XTi EP recording system, released in May 2009. CardioICE synchronizes EP signal data and procedure information with real-time ultrasound images.


 May 11, 2009
May 11, 2009











