June 25, 2009 – Smiths Medical today launched its new BCI SPECTRO2 Pulse Oximetry Portfolio, designed for a variety of patient care settings.
June 24, 2009 – Data presented today as a Hot Line session at the EUROPACE 2009 congress on the XPECT clinical trial, sponsored by Medtronic Inc., shows that the Medtronic Reveal XT Insertable Cardiac Monitor (ICM) reliably identifies patients with AF (sensitivity of 96.1 percent) and correctly confirms the absence of AF in patients (negative predictive value of 97.4 percent).
June 24, 2009 – CorNova Inc. said this week it received CE mark approval for its Valecor Platinum Coronary Stent System, a next generation cobalt-chromium bare metal stent.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
June 24, 2009 – CircuLite Inc. today announced the publication of positive data from its ongoing CE mark clinical trial for the Synergy Pocket Micropump for chronic heart failure in the Journal of the American College of Cardiology.
June 24, 2009 - NDS Surgical Imaging (NDSsi) announced it acquired a wireless video technology that extends its current imaging capabilities in the operating room (OR) and beyond. By integrating this new technology with its medical visualization and informatics platforms, the company plans to develop a compelling array of wireless solutions for surgical, endoscopy and interventional suites.
June 23, 2009 – Boston Scientific Corp.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
June 23, 2009 - Today, Medtronic Inc. announced the first worldwide enrollments in the MORE-CARE (Monitoring Resynchronization in Cardiac Patients) trial, which will compare two disease management strategies for heart failure patients treated with an implantable device for cardiac resynchronization therapy.
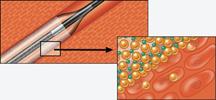
The focus on balloon angioplasty largely slipped from the interventional limelight a decade ago after the introduction of stents, but today there is some renewed interest with the advent of new balloon technology.
June 23, 2009 – A significant study was released today demonstrating the efficacy of interactive patient care technology on improving outcomes in heart failure care.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
June 23, 2009 - WaveMark Inc. announced today its clinical inventory management solution, WaveMark CIMS (clinical inventory management system), has been selected by Tufts Medical Center to manage the hospital’s high value, specialty inventory.
June 23, 2009 – Abbott said today it received CE mark (Conformite Europeenne) for its next-generation Xience PRIME Everolimus-Eluting Coronary Stent System for the treatment of coronary artery disease. The company plans to launch Xience PRIME in a broad size matrix with lengths up to 38 mm in Europe in the third quarter.
June 23, 2009 - On June 12, 2009, FUJIFILM Medical Systems USA Inc., announced that Amerinet, one of the leading healthcare group purchasing organizations (GPOs) in the United States, added Synapse cardiovascular and Synapse RIS to its existing Fujifilm contracts.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
June 23, 2009 – AccessClosure yesterday launched its newest addition to the Mynx Vascular Closure family, the Mynx M5, which can be delivered directly through a small 5 Fr. cardiovascular sheath without enlarging the arterial hole or traumatizing the surrounding tissue in the process.
June 23, 2009 - Kensey Nash Corp. today said the ThromCat XT Thrombectomy Catheter System was granted CE marking approval, allowing for the sale of the product throughout the European Economic Area (EEA).
June 22, 2009 - Medical Corp. said last week the ambulance service RAV Gooi en Vechtstreek, in Hilversum, the Netherlands, is the first EMS organization to equip its ambulances with a new system that allows rescuers to defibrillate a heart without the need to stop chest compressions.

 June 24, 2009
June 24, 2009









