January 13, 2011 – St. Joseph's Hospital in Tampa, Fla., and the Baylor Jack and Jane Hamilton Heart and Vascular Hospital in Dallas, Texas, are among the first U.S. hospitals to treat patients with atrial fibrillation (A-fib) using a new freezing balloon technology. The minimally-invasive catheter system is the first of its kind in the United States and was approved by the U.S.
January 13, 2011 – A new robotic navigation system has both received the CE mark and undergone its first successful cardiac ablation procedure. The Vdrive robotic navigation system, from Stereotaxis, provides a companion to magnetic navigation.
January 12, 2011 – A new, next-generation vascular closure device has been launched in the United States. The Mynx Cadence Vascular Closure Device (VCD), from AccessClosure, offers physicians smoother device deployment while maintaining all the benefits of the original Mynx.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
January 12, 2011 – The Society for Cardiovascular Angiography and Interventions (SCAI) has published a paper on the future trends and developments in the cardiac catheterization laboratory and interventional vascular suite.
Two applications will help clinicians perform interventions with confidence and accuracy.
Vital Images is a leader in advanced visualization solutions worldwide, partnering with several leading companies. Its technology is available anywhere, anytime, on the cloud, an iPad, laptop computer, in the home or office.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
January 11, 2011 - nContact Surgical, a cardiac ablation device manufacturer, is changing its name to nContact to reflect the trend away from surgery toward percutaneous and minimally invasive procedures.
January 11, 2011 – TriReme Medical has signed an exclusive distribution and sales agreement with Century Medical for the Japanese market. Century Medical will sell TriReme’s compete line of advanced peripheral and coronary balloon dilatation catheters.
January 11, 2011 – Stereotaxis has named Siemens as the non-exclusive reseller of the Odyssey Enterprise Cinema solutions. These products are designed to enhance patient care by enabling hospitals to remotely access integrated data from procedures throughout hospital networks and around the world.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
January 11, 2011 – A new vascular access system is available in the United States and Europe. The SmartNeedle Vascular Access System, by Vascular Solutions, consists of a hand-held monitor and one-time use needles designed to provide auditory ultrasound-guided access during catheterization procedures. The Doppler guided needle access system was acquired from Escalon Medical in May 2010.
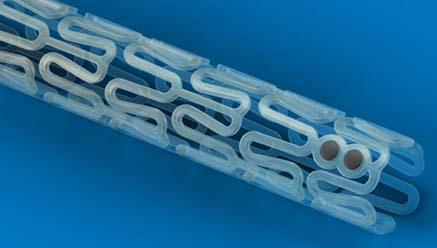
January 10, 2011 – European CE mark approval was announced today for the Abbott, Absorb drug-eluting bioresorbable coronary stent. It is the first bioresorbable stent to gain regulatory approval anywhere in the world. Full commercial launch in Europe is planned by the end of 2012.
January 7, 2011 – A new study from The Ohio State University found that Protandim can suppress intimal hyperplasia, the over-proliferation of cells that line the walls of blood vessels. The adverse blood vessel blockage often limits the effectiveness of several types of vascular surgery.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
January 7, 2011 – The United States Patent and Trademark Office (USPTO) has granted an additional patent expanding protection of endothelial progenitor cell (EPC) capture technology. It applies to OrbusNeich’s Genous EPC technology.
January 7, 2011 – A new access device is now available in cath labs across the United States. The Arstasis One Access Device, from Arstasis, is the first commercially available alternative to the Seldinger Technique in 52 years. Previously, only a select number of physicians participating in Arstasis clinical trials and select pre-launch registries have been able to use the device.
January 6, 2011 – The Heart Rhythm Society (HRS) recently hosted its inaugural two-day research forum to identify opportunities and challenges to advancing research in electrophysiology (EP).


 January 13, 2011
January 13, 2011












