GE Healthcare unveiled two recently U.S. Food and Drug Administration (FDA)-cleared angiography systems offering mobility and advanced imaging for interventional cardiology at the American College of Cardiology (ACC) 2012.
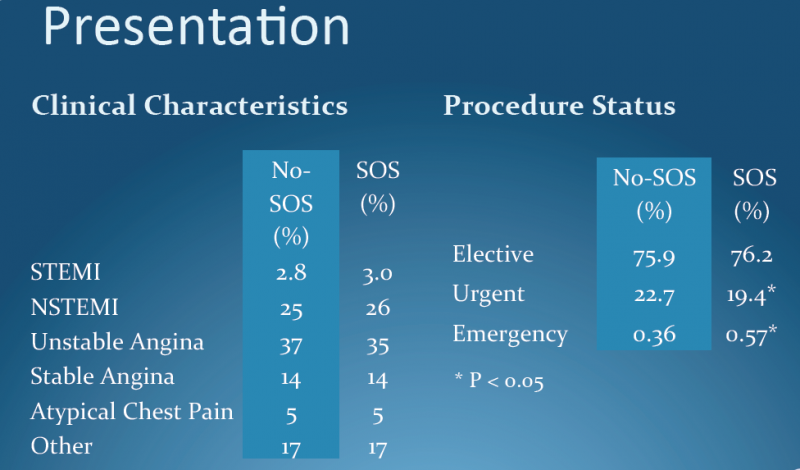
New evidence shows that with appropriate preparation, angioplasty can be safely and effectively performed at community hospitals without on-site cardiac surgery units. This was according to data presented from the CPORT-E trial during the American College of Cardiology's (ACC) 61st Annual Scientific Session this week in Chicago.
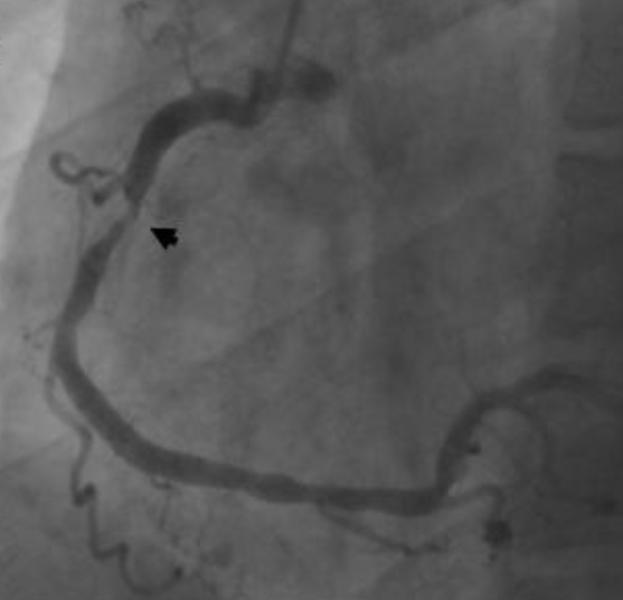
Coronary angiography is unable to accurately predict the severity of vessel narrowing, suggesting fractional flow reserve (FFR) functional tests should be added to help determine if a patient needs revascularization. This was according to research presented from the IRIS FFR-DEFER trial at the American College of Cardiology's (ACC) 61st Annual Scientific Session this week in Chicago.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

The cost to place an implantable cardioverter-defibrillator (ICD) increased by $844 per case after a new requirement from the Centers for Medicare and Medicaid Services (CMS) went into effect in February 2010, which has added little additional benefit for patients. This was according to research presented during the American College of Cardiology’s 61st Annual Scientific Session in Chicago this week.

March 27, 2012 – Researchers found the antiplatelet drug abciximab significantly decreased damage to the heart muscle in patients with ST-segment-elevation myocardial infarction (STEMI), the most severe type of heart attack. Results of INFUSE-AMI trial were presented at the American College of Cardiology’s 61st Annual Scientific Session and published simultaneously in the March 25 online issue of the Journal of the American Medical Association. Researchers also found that clot aspiration did not significantly reduce the damage to the heart muscle.
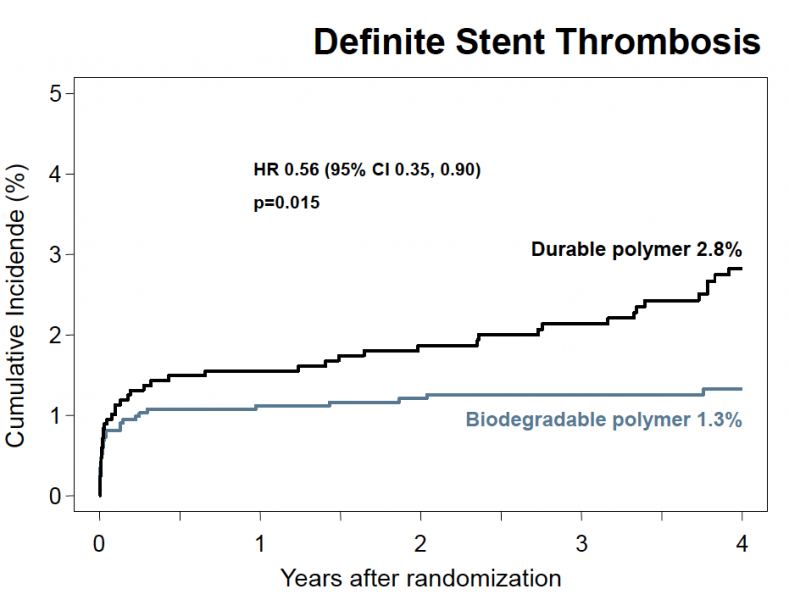
Biodegradable polymer drug-eluting stents (DES) provide better long-term safety and efficacy than durable polymer DES, according to findings from an analysis of three major clinical trials — ISAR-TEST 3, ISAR-TEST 4 and LEADERS. The data were presented at at the American College of Cardiology’s 61st Annual Scientific Session.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
March 26, 2012 — ScImage Inc. launched a new cloud electrocardiogram (ECG) management service as an extension of its PicomCloud cloud picture archiving and communications system (PACS) at the American College of Cardiology's (ACC) 61st Annual Scientific Session in Chicago.
March 26, 2012 - New research shows that the addition of two magnetic resonance imaging (MRI) sequences to a common MR angiography technique significantly improves detection of pulmonary embolism, a potentially life-threatening condition traditionally diagnosed through computed tomography (CT). Results of the study are published online in the journal Radiology.
March 26, 2012 — Itamar-Medical unveiled its cardiovascular healthcare assessment platform, EndoPAT-MF, at the American College of Cardiology’s 61st Annual Scientific Session (ACC.12) in Chicago. The multifunction EndoPAT-MF now features five major diagnostic tools in a single system.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
March 26, 2012 — GE Healthcare introduced the 510(k)-pending Discovery CT750 HD FREEdom Edition at the opening of American College of Cardiology’s 61st Annual Scientific Session in Chicago. Addressing the main challenges of cardiac imaging – coronary motion, high heart rates, calcium blooming, plaque composition and accurate myocardial perfusion – the FREEdom Edition is designed to provide a new level of cardiac computed tomography (CT) performance and to help physicians best serve patients.
March 26, 2012 — Maquet announced positive long-term mortality results from the Balloon Pump-Assisted Coronary Intervention Study (BCIS-1), which evaluated the effect on major complications and mortality of elective use of an intra-aortic balloon pump (IABP) in patients with low ejection fraction undergoing high-risk percutaneous coronary intervention (PCI).
March 23, 2012 — New findings from a research study led by Scripps Translational Science Institute (STSI), a collaborative program between Scripps Health and the Scripps Research Institute (TSRI), shows a new blood test may be useful in helping doctors predict who is at risk for an imminent heart attack.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
March 23, 2012 — Researchers using the VAP (vertical auto profile) Cholesterol Test to isolate lipid subfractions and assess a new anti-flushing agent have reported significant baseline reductions in the cholesterol content of lipoproteins across the entire VLDL to LDL density range in dyslipidemic patients.
March 23, 2012 — Improving the ability to diagnose cardiac disease with advanced ultrasound imaging, Toshiba America Medical Systems Inc. will showcase the newest additions to its ultrasound product line, Aplio 500 and Aplio 300, at the American College of Cardiology’s annual meeting March 24-27, 2012 in Chicago. Toshiba will also be introducing new enhancements to its Aplio Artida flagship cardiac ultrasound.
March 23, 2012 — ScImage Inc. announced that all new ScImage PACS installations will now include off-site disaster recovery services free of charge.


 March 27, 2012
March 27, 2012











