Internet-based telemedicine systems appear to lead to more appropriate and effective pharmacotherapy, better blood pressure control and an overall reduction in cardiovascular risk compared to conventional, periodic office visits, according to research presented this week at the American College of Cardiology (ACC) Annual Scientific Session in Chicago.
A late-breaking stem cell trial presented at the American College of Cardiology (ACC) Annual Scientific Session showed only minor improvement in heart pumping capability between baseline and six months following treatment.

Two-year follow-up data from the pivotal PLATINUM Workhorse trial comparing the safety and effectiveness of the Promus Element and Xience V everolimus-eluting coronary stents demonstrated superior efficacy of the Promus. The results were presented at the American College of Cardiology (ACC) Annual Scientific Session by Gregg W. Stone, M.D., professor of medicine and director of research and education at the Center for Interventional Vascular Therapy at Columbia University Medical Center/New York-Presbyterian Hospital. He was the global principal investigator of the trial.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
March 29, 2012 — Non-cardiac co-morbidities such as chronic obstructive pulmonary disease, chronic kidney disease and frailty are the main predictors of late mortality after transcatheter aortic valve implantation (TAVI), suggesting that patients with these conditions merit closer evaluation and follow-up, according to research presented at the American College of Cardiology’s (ACC) 61st Annual Scientific Session.
March 29, 2012 — In the second year of an ongoing trial, the Resolute zotarolimus-eluting stent (Medtronic) achieved a low rate of stent thrombosis, cardiac death, target vessel heart attack and target lesion revascularization years, according to research presented at the American College of Cardiology’s (ACC) 61st Annual Scientific Session.
March 29, 2012 — Adding vorapaxar, an investigational platelet blocker, to standard antiplatelet therapy significantly reduces the risk of recurrent cardiovascular events in a specific group of patients, but also increased the risk of severe bleeding, including intracranial bleeding. This was according to research in the TRA 2°P-TIMI 50 Trial presented during the American College of Cardiology (ACC) 61st Annual Scientific Session in Chicago.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
March 29, 2012 — A three-pronged intervention in Brazilian public hospitals significantly improved physician adherence to evidence-based protocols for treating acute coronary syndrome (ACS), according to data from the BRIDGE-ACS trial. The research was presented during the American College of Cardiology’s (ACC) 61st Annual Scientific Session this week in Chicago.
March 29, 2012 — VectraCor Inc. announced at the American College of Cardiology’s 61st Annual Scientific Session (ACC) in Chicago that it received U.S. Food and Drug Administration (FDA) approval for the VectraplexECG System with VectraplexAMI. It is a stand-alone cardiac monitor/electrocardiography (ECG) machine with a cardiac electric biomarker (CEB) for real-time detection of ECG changes that may be indicative of an acute myocardial infarction (AMI) plus the capability to derive a 15-lead ECG.
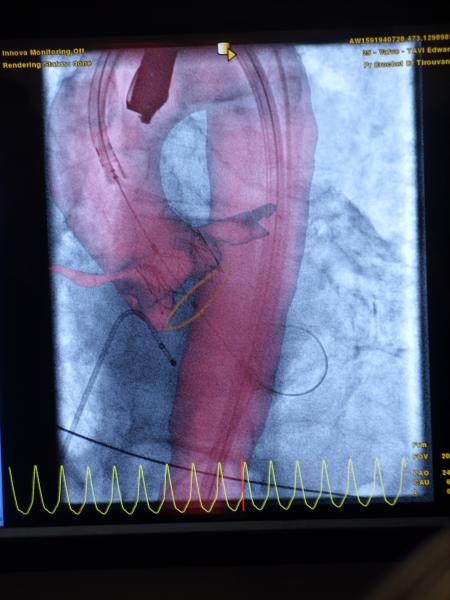
The American College of Cardiology (ACC) 2012 Scientific Session, held March 24-27 in Chicago, was the first major cardiology show this year for vendors to display their latest innovations. A couple of key trends were evident on the show floor – new technology to support trans-aortic valve replacement (TAVR) and the launch of new cardiovascular image and information systems (CVIS) to support healthcare’s proposed Stage 2 meaningful use (MU) requirements.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
March 29, 2012 — St. Jude Medical Inc. announced it has received U.S. Food and Drug Administration (FDA) clearance for multiple enhancements to its PressureWire Fractional Flow Reserve (FFR) measurement guidewire. FFR measurement identifies the severity of narrowings in the coronary arteries and allows for a more effective assessment of coronary lesions, or blockages, resulting in more accurate diagnosis and improved appropriate treatment of coronary artery blockages.
March 29, 2012 — Siemens Healthcare recently expanded its solutions portfolio by offering the RaySafe i2 personal dosimetry system as an accessory for all Siemens Artis zee angiography systems. By using RaySafe i2 during imaging procedures, medical personnel obtain real-time information regarding their levels of radiation exposure, enabling them to take immediate steps to minimize exposure and establish a high-functioning radiation safety culture within their hospital.

Two large clinical trials were presented in the late-breaking clinical trials session at the American College of Cardiology's (ACC) 61st Annual Scientific Session this week that indicate coronary computed tomography angiography (CCTA) used as a tool to evaluate patients with chest pain in the emergency department is safe, time-efficient and cost-effective, compared to the current standard approach.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Agfa HealthCare's new modular version of its cardiovascular information system (CVIS), Impax CV12, was unveiled at the American College of Cardiology (ACC) Annual Scientific Session this week in Chicago.
GE Healthcare unveiled two recently U.S. Food and Drug Administration (FDA)-cleared angiography systems offering mobility and advanced imaging for interventional cardiology at the American College of Cardiology (ACC) 2012.
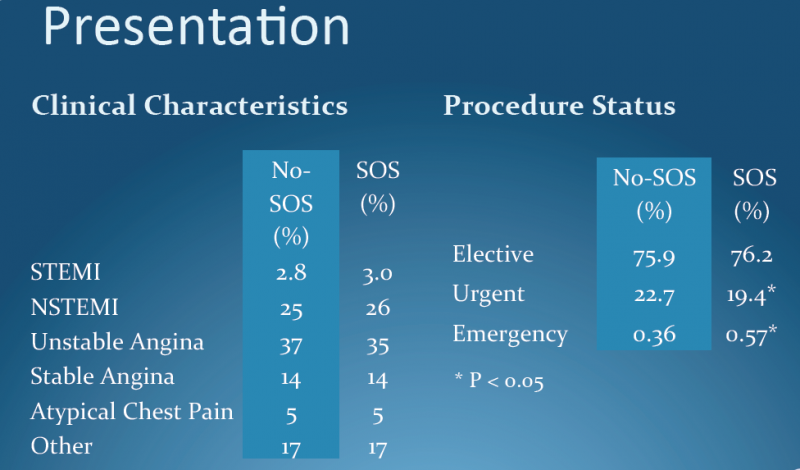
New evidence shows that with appropriate preparation, angioplasty can be safely and effectively performed at community hospitals without on-site cardiac surgery units. This was according to data presented from the CPORT-E trial during the American College of Cardiology's (ACC) 61st Annual Scientific Session this week in Chicago.

 March 29, 2012
March 29, 2012












