April 17, 2012 — Isansys Lifecare Ltd., provider of complete real-time physiological patient data services and systems, announced that it has achieved CE certification for its LifeTouch Patient Surveillance System, comprising the LifeTouch HRV011 intelligent body-worn wireless sensor and associated Patient Gateway. The LifeTouch is the world’s first cloud-ready medical device of its kind.
April 17, 2012 — St. Jude Medical Inc. announced the first implant in its Accent MRI Pacemaker and Tendril MRI Lead IDE Study. The ultimate goal of the study is to determine if patients with these devices can safely undergo full-body, high-resolution magnetic resonance imaging (MRI) scans to better accommodate their medical needs.
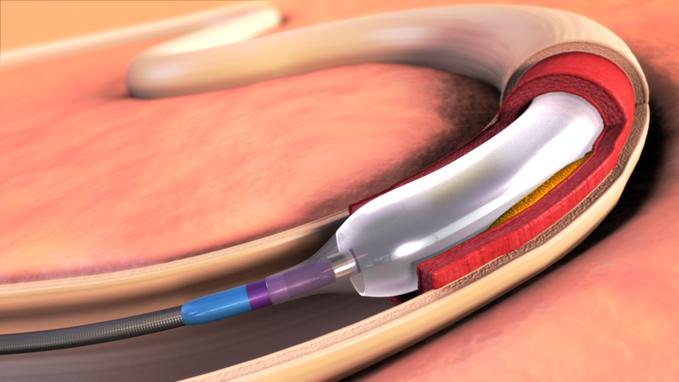
April 17, 2012 –– Expanding its commitment to developing innovative treatments for cardiovascular disease and the evidence to support their adoption, Medtronic announced the start of the Medtronic IN.PACT SFA II study, the company’s first U.S. clinical trial for its line of IN.PACT drug-eluting balloons.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
April 17, 2012 — Radius LLC is expanding its unique pay-per-study RIS/PACS model to include the option of on-demand 3-D imaging reconstruction.
April 17, 2012 - Approximately 6 million Americans have brain aneurysms, a condition that occurs when a weak or thin spot develops on a blood vessel in the brain causing it to balloon. Often, these do not cause symptoms and go undetected, but every year an estimated 30,000 Americans experience a ruptured aneurysm that bleeds into the brain causing a life threatening injury. Immediate medical treatment is necessary to prevent stroke, nerve damage or death, and includes surgery or coiling. Coiling is an approach that blocks blood flow to the aneurysm by filling it with platinum coils. While less invasive than surgery, the likelihood of future aneurysm recurrence and subsequent treatment is higher with coiling. In an effort to lower the risk for repeat aneurysm treatment after coiling, Northwestern Medicine researchers are examining a new type of gel-coated coil to determine if it is more effective than the standard bare coils in preventing aneurysm recurrence.
April 12, 2012 – Abbott announced approval from the Japanese Ministry of Health, Labor and Welfare (MHLW) for the next-generation Xience Prime Everolimus-Eluting Coronary Stent System for the treatment of coronary artery disease. XIENCE PRIME, which uses the same drug and biocompatible polymer as the XIENCE V Everolimus Eluting Coronary Stent System, features an enhanced stent design and a delivery system designed for greater flexibility, ideal radial strength, excellent longitudinal strength and more accurate stent placement. With this approval in Japan, XIENCE PRIME is available in all of the major markets worldwide, including the United States, Europe, China, India and other countries in the Asia-Pacific and Latin America regions.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
April 17, 2012 - On April 9, 2012, the FDA's Center for Devices and Radiological Health (CDRH) launched its second version of the Innovation Pathway, called "Innovation Pathway 2.0." This program offers new and modified tools and methods to deepen collaboration between the FDA and innovators early in the process, prior to pre-market submission, with the goal of making the regulatory process more efficient and timely.
April 17, 2012 – SynCardia Systems Inc., manufacturer of the world’s first and only FDA-, Health Canada- and CE-approved Total Artificial Heart, announced that the U.S. Food and Drug Administration (FDA) has approved a Humanitarian Use Device (HUD) designation for the SynCardia temporary Total Artificial Heart to be used for destination therapy in addition to its current PMA approval as a bridge to transplant.
April 16, 2012 — Medic Vision Imaging Solutions Ltd. announced that within six months of clinical use in the United States, its SafeCT image enhancement system has delivered diagnostic image quality to more than 20,000 CT (computed tomography) studies acquired with low-dose protocols.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
April 16, 2012 - 480 Biomedical announced that it has initiated the first human trial of its groundbreaking Stanza self-expanding bioresorbable scaffold for the treatment of peripheral artery disease (PAD) in the superficial femoral artery (SFA). The Stanza scaffold restores blood flow to the leg by propping open the diseased artery during the critical healing period, similar to conventional metal stents, but then is resorbed by the body. Observations from the first patients in the STANCE trial were reported on April 14 at the 34th Charing Cross International Symposium in London.
April 16, 2012 - Prime Healthcare Services (PHS), a hospital management company based in Ontario, Calif., currently owns and operates 16 acute-care hospitals. In February 2012, PHS signed a master agreement with Infinitt North America to replace PACS and cardiology PACS at several of its California hospitals.
April 15, 2012 - St. Jude Medical Inc., a global medical device company, announced CE Mark Approval of the Ellipse implantable cardioverter defibrillator (ICD). Designed with feedback from more than 200 physicians from around the world, the Ellipse ICD provides the benefits of advanced features and power in the industry’s smallest high-energy ICD.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
April 16, 2012 — Philips Healthcare announced it is collaborating with Brainlab AG to create a comprehensive intra-operative magnetic resonance imaging (MRI) solution with the goal of streamlining neurosurgery procedures. Ingenia MR-OR is based on Philips’ digital broadband Ingenia MRI system, 1.5T and 3.0T, and is designed to be combined with Brainlab’s integrated operating room (OR) solutions.
April 13, 2012 — Researchers at The Ohio State University (OSU) Wexner Medical Center have successfully used nanotechnology to target a protein that plays a key role in atherosclerosis and inflammation, and say the study is an important advance toward using immunotherapy to simultaneously diagnose and treat cardiovascular disease.
April 12, 2012 — Several innovative cardiovascular and radiology technologies were featured in Medical Device and Diagnostic Industry (MD+DI) magazine’s April issue, which also announced the finalists in the 2012 Medical Design Excellence Awards (MDEA) competition.


 April 17, 2012
April 17, 2012












