April 17, 2012 — Isansys Lifecare Ltd., provider of complete real-time physiological patient data services and systems, announced that it has achieved CE certification for its LifeTouch Patient Surveillance System, comprising the LifeTouch HRV011 intelligent body-worn wireless sensor and associated Patient Gateway. The LifeTouch is the world’s first cloud-ready medical device of its kind, and the first to be certified as a Class IIa medical device under the European Medical Device Directive (MDD). The LifeTouch system is now ready for clinical use in the European Union (EU) and other countries that recognize the CE mark.
The LifeTouch System provides continuous real-time information for automated patient surveillance, early warning scores and track and trigger indicators, and for predicting adverse events. Through its low cost and highly scalable architecture, LifeTouch offers the opportunity for continuous surveillance of all patients regardless of whether they are in hospital, other care facilities or at home, helping to reduce costs associated with avoidable patient deterioration, medical errors, readmissions to intensive care wards and admissions (and re-admissions) to hospital.
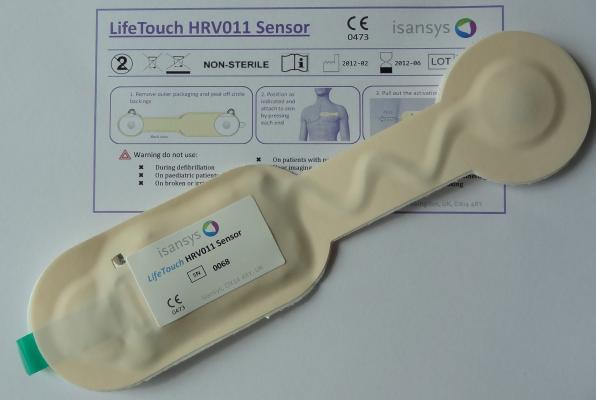
The ultra-low profile and lightweight LifeTouch HRV011 sensor measures and analyzes the electrocardiogram (ECG) signal of every heartbeat in real-time to provide data for heart rate variability (HRV) methods. Robust algorithms in the HRV011 extract key ECG parameters from which heart rate and respiration rate are continuously derived. These key vital signs, together with the HRV data, may be viewed directly via a local network on the Patient Gateway web server interface, or forwarded into a secure cloud-based electronic patient record that is updated continuously and in real-time.
“This CE certification marks a major milestone on the road to global pervasive adoption of these kinds of essential devices. Our LifeTouch system – the world’s first cloud-ready medical device and now, the first single-use smart patch device to achieve a CE mark – is in the vanguard and now ready for full-scale deployments,” said Keith Errey, CEO of Isansys Lifecare.
Rebecca Weir, Isansys director of business development, added: “We are now focused on another significant industry first – building the Isansys Healthcare Cloud to deliver the scalability, accessibility and affordability benefits of cloud-based technology to the clinical market for the first time. We are working to create this brand new ecosystem and secure clinical network infrastructure that will match the very special characteristics and requirements of healthcare providers and patient care environments.”
The LifeTouch HRV011 sensor is a single-use, semi-disposable device with low-cost body contacting parts. Key target markets for Isansys outside of Europe include developing economies where the system offers healthcare providers the ability to leapfrog many of the expensive medical devices currently in use. The LifeTouch system incorporates enormous intelligence, providing scope for additional functionality in the future that will open up vast new opportunities for low-cost screening and targeted monitoring of specific cardiac conditions. The system is covered by patent applications relating to construction, use and functionality, and is available through the Isansys service offerings or under license from Isansys.
For more information: www.isansys.com


 January 15, 2026
January 15, 2026 









