September 28, 2012 — Boston Scientific Corp. announced it has received U.S. Food and Drug Administration (FDA) clearance for the Emerge percutaneous transluminal coronary angioplasty (PTCA) balloon dilatation catheter, and has begun marketing the device in the United States.
Medtronic Inc. announced it has received CE mark for its Medtronic CoreValve Evolut 23 mm valve, its latest self-expanding transcatheter aortic valve implantation (TAVI) system.
Boston Scientific has launched the Promus Element Plus BTK (Below The Knee) everolimus-eluting stent system in European countries for the treatment of certain severe peripheral artery lesions. The drug-eluting stent (DES) has CE mark approval for both monorail and over-the-wire (OTW) versions and will be available immediately.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The average age of installed magnetic resonance imaging (MRI) scanners in the United States has increased from eight years in 2007 to 10.9 years in 2011, according to a new market research report by IMV Medical Information Division.
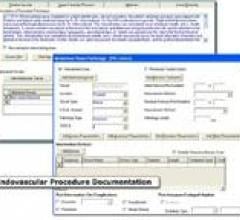
The American College of Cardiology (ACC) has certified Lumedx software as fully interoperable between four ACC NCDR registries. Lumedx, a leading provider of integrated cardiovascular imaging and information systems, is currently the only software vendor offering this level of interoperability.

Abbott Vascular began its international launch of the Absorb coronary stent, the world's first drug-eluting bioresorbable vascular scaffold (BVS). It is now widely available across Europe and parts of Asia Pacific and Latin America. The Absorb is a first-of-its-kind device for the treatment of coronary artery disease (CAD).
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
September 25, 2012 — Patients are often aggressively treated with anticoagulants, or blood thinners, to help prevent pulmonary emboli from forming, but a study published in the September 2012 issue of the Journal of the American Academy of Orthopaedic Surgeons indicates that some blood clots being identified by today's sensitive testing methods may not require aggressive treatments.

Sept. 25, 2012 — America's healthcare system has become too complex and costly to continue business as usual, says a new report from the Institute of Medicine. Inefficiencies, an overwhelming amount of data and other economic and quality barriers hinder progress in improving health and threaten the nation's economic stability and global competitiveness, the report says. However, the knowledge and tools exist to put the health system on the right course to achieve continuous improvement and better quality care at lower cost, added the committee that wrote the report.

A new survey reveals that cardiologists around the United States are seeing more patients than ever before, yet performing fewer advanced nuclear tests on those patients on average. The survey cites a change in the approach to the delivery of cardiovascular care, as well as continued concerns about economic conditions, as reasons for the decline.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

According to Millennium Research Group (MRG), the global authority on medical technology market intelligence, the saturated U.S. X-ray market will grow slowly to reach a value of $2.8 billion by 2016. Significant growth will be seen in digital replacement systems in all segments of the market.
The new U.S. Food and Drug Administration Safety and Innovation Act (FDASIA) imposes many Medical Device Establishment Registration and Listings requirements, effective Oct. 1, 2012. To assist the medical device industry, Registrar Corp has provided the following list of top 10 things that medical device professionals need to know regarding FDASIA:

September 24, 2012 — Surgically inserted cardiac devices play an important role in treating certain heart problems. In fact, an estimated 400,000 devices, including pacemakers and cardioverter defibrillators, are implanted each year in the United States. Still, selecting patients in whom these devices will provide the most benefit can be challenging.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
September 24, 2012 — Bio DG announced that its patent for a novel metal system, to use as biodegradable material in developing implantable medical devices, was granted by the U.S. Patent Office on Aug. 21, 2012. Bio DG's biodegradable alloys are primarily metallic and provide high strength when first implanted, then gradually erode in a predictable and biocompatible manner.
September 21, 2012 — Endologix Inc. announced this month it received CE mark for the current version of the Nellix EndoVascular Aneurysm Sealing System for the treatment of patients with abdominal aortic aneurysms (AAA).
September 21, 2012 — Following the success of Biotronik’s Pulsar-18 0.018-inch, 4 French platform self-expanding stent system, the company has introduced its Pulsar stent technology in a 0.035-inch, 6 French platform.


 September 28, 2012
September 28, 2012