The environment surrounding a medical imaging system can now be monitored with Toshiba America Medical Systems Inc.’s e-Watch. This integrated solution monitors power, temperature and humidity in the room in which the imaging system resides, ensuring optimal performance and uptime.

November 20, 2012 — The American College of Cardiology (ACC), in collaboration with several other professional societies, has released the first comprehensive consensus document outlining clinical considerations for ordering and interpreting tests for troponin, a protein that indicates injury to the heart muscle.
Image Wisely, in collaboration with the Society of Nuclear Medicine and Molecular Imaging (SNMMI), SNMMI Technologist Section and American Society of Nuclear Cardiology (ASNC), has created easily accessible online educational materials to help providers use the lowest radiopharmaceutical dose necessary to perform nuclear medicine exams.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Lantheus Medical Imaging Inc. and two medical isotope producers have agreed to work together to help ensure supplies of potentially life-saving nuclear medicine using molybdenum-99 (Mo-99) sourced from low enriched uranium (LEU).
November 15, 2012 – GE Healthcare Canada announced Health Canada clearance of its new cardiac imaging platform, the Discovery* CT750 HD FREEdom Edition. Addressing the main challenges of cardiac imaging – coronary motion, calcium blooming, plaque composition and accurate myocardial perfusion – the FREEdom Edition is designed to provide a new level of cardiac CT performance.
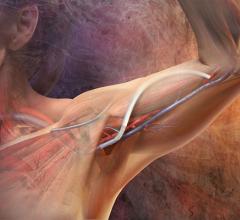
W. L. Gore and Associates has announced the commercial availability of its new 10-cm configuration of the Gore Hybrid Vascular Graft. The new configuration allows for access to deeper vessels, which expands the treatable patient population and offers exceptional ease of use for physicians performing vascular procedures.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

November 20, 2012 — More than 570 providers identify McKesson, Merge, Philips and Siemens as the top vendors in terms of overall provider satisfaction and adoption, according to the recently released KLAS study, Cardiology IT 2012: Fitting the Pieces Together.
Medtronic Inc. announced the first treatment of a U.S. patient in its global, multicenter, randomized clinical trial comparing the Medtronic CoreValve system with surgical aortic valve replacement in patients with severe aortic stenosis who are at intermediate risk to undergo open-heart surgery. The Medtronic CoreValve Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVI) Trial is evaluating the minimally-invasive CoreValve system in less-sick patients who typically are treated with open-heart surgical aortic valve replacement today.
Biotronik announced that high profile medical centers across the country are implanting the new Evia HF-T triple-chamber cardiac resynchronization therapy (CRT) pacemaker.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

Ischemic heart disease affects nearly 10 million Americans, and remains the leading cause of death among U.S. adults. This condition is most often due to a build-up of fat or plaque in the heart’s arteries that can reduce critical blood flow to the heart and often other parts of the body as well. With appropriate medical care, this condition remains stable in the vast majority of patients with few or no symptoms and the ability to pursue normal activities without limitations. Still, ongoing medical therapy and careful monitoring are essential to prevent heart attack and untimely death and should be based on strong scientific evidence and individual patient preferences.
Cordis Corp. announced that the U.S. Food and Drug Administration (FDA) has approved the S.M.A.R.T. Control Vascular Stent Systems for use in the superficial femoral artery (SFA) and/or the proximal popliteal artery (PPA). The S.M.A.R.T. Stent, which has been approved for peripheral indication in international markets since 1999, is now the first stent in the United States with both Iliac and SFA indications.
CardioNet Inc. announced the launch of its new wireless event monitor, wEvent.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

The introduction of hybrid technology — positron emission tomography/computed tomography (PET/CT) and single-photon emission computed tomography (SPECT)/CT -— has revolutionized the imaging world. This technology allows the combination of the exquisite anatomic details provided, for example, by CT, with the important and much needed functional, physiologic or metabolic information provided by molecular imaging.
New endovascular grafting techniques to repair tissue following abdominal aortic aneurysm rupture are generating growing enthusiasm among vascular surgeons, and a number of those new treatment options were presented at the 39th VEITHsymposium held in New York City Nov. 14 to 18.
Stryker announced the global commercial launch of the new Trevo ProVue Retriever. The Trevo ProVue Retriever is the first clot removal device fully visible during the procedure for precise positioning within the clot and optimized clot retrieval in patients experiencing acute ischemic stroke. Previous technology only provided visibility to the edges of the device.

 November 20, 2012
November 20, 2012