More than half of patients treated for claudication or critical limb ischemia (CLI) with stenting of the superficial femoral artery (SFA) had primary patency at five years, says a recent study.
Featuring hovercraft-like C-arm movement and the unique Access Halo, the WorkRite technology on Toshiba America Medical Systems Inc.’s Infinix-i cardiovascular X-ray systems makes interventional procedures easier for clinicians and improves patient care.

Tryton Medical Inc. announced the completion of enrollment in the TRYTON Pivotal U.S. Food and Drug Administration (FDA) IDE trial evaluating the Tryton Side Branch Stent.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Volcano Corporation announced that it has entered into a definitive agreement to acquire Sync-Rx Ltd., a privately-held company based in Israel that develops advanced software applications that optimize and facilitate transcatheter cardiovascular interventions using automated online image processing. It is anticipated the transaction will close within the next 30 days.
December 6, 2012 — Neusoft Medical Systems Co. Ltd., a wholly owned subsidiary of Neusoft Corp., announced this week that its NeuViz 64 multi-slice computed tomography (CT) scanner has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
December 6, 2012 — Daiichi Sankyo Inc. and Eli Lilly and Co. announced results of two retrospective studies comparing rates of readmission for subsequent heart attack and initial hospitalization costs among patients with acute coronary syndromes (ACS) treated with a percutaneous coronary intervention (PCI) and antiplatelet therapy.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Giving clinicians a more complete picture while improving safety during interventional procedures, Toshiba America Medical Systems Inc. introduces Spot Fluoroscopy for its Infinix-i systems. Enabling quicker diagnoses and lower dose, clinicians can observe a target region of anatomy using Spot Fluoro’s live fluoroscopy while viewing the last image hold (LIH) surrounding area.
The U.S. Food and Drug Administration (FDA) granted premarket approval (PMA) for the HeartWare Ventricular Assist System (VAS) designed as a bridge to cardiac transplantation.

As electronic medical records become more sophisticated and healthcare moves to an increasingly paperless system, electrocardiogram (ECG) integration has become a priority for many hospitals. For hospitals that have been using ECG management systems, many are upgrading to newer systems that offer better integration with their ECG systems, interoperability with other software systems, or offer Web-access and smart phone access.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
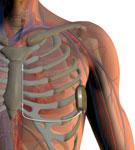
The U.S. Food and Drug Administration (FDA) in October granted Boston Scientific Corp. regulatory approval for its S-ICD System, the world’s first commercially available subcutaneous implantable cardioverter defibrillator (S-ICD). The system sits entirely just below the skin without the need for implantable lead to be placed inside the heart. This offers patients an alternative to transvenous ICDs, which require leads to be placed in the heart itself.
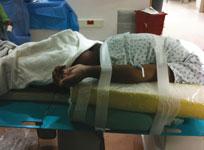
Radial access has been a standard for most patient cases for several years at both the University of Illinois at Chicago (UIC) Medical Center and the Jesse Brown VA Medical Center. The centers are located within two blocks of each other, and the two staffs work very closely. Part of their coordination has been developing training programs and best practices and protocols for radial artery access techniques.
GE Healthcare announced at RSNA 2012 receipt of U.S. Food and Drug Administration (FDA) clearance for its advanced imaging tool, FlightPlan for Liver. Developed to help make intricate liver embolization procedures simpler, FlightPlan for Liver has been commercially available in Europe, Latin America and Asia since 2011, with more than 30 installations in more than 10 countries.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Bracco Diagnostics Inc. is voluntarily initiating a Class I recall of nine lots of Isovue (iopamidol injection) pre-filled power injector syringes (Isovue PFS, to be used in combination with Stellant CT [computed tomography] injection systems) due to the presence of visible particles in syringes observed at the end of standard stability studies on retained samples. These products were distributed to wholesalers and distributors nationwide.

Results of a study investigating the effects of smoking on the antiplatelet medications clopidogrel and prasugrel were presented at the 24th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium.
A large observational study found the increased risk associated with dual antiplatelet therapy cessation after percutaneous coronary intervention (PCI) is tied to patient non-adherence, as opposed to physician-recommended discontinuation. Results of the PARIS registry were presented at the 24th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium.

 December 07, 2012
December 07, 2012