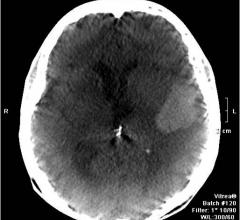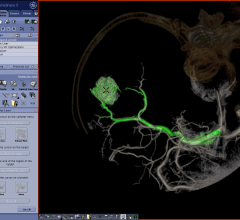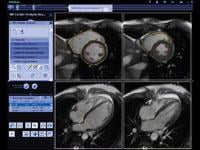
Increasingly software applications are providing many of the major advancements in magnetic resonance imaging (MRI) rather than hardware. In recent years, this has translated into software that enables physicians to do more in less time, extract more diagnostic information from imaging exams and provide more accurate quantification. While a hospital may not spend millions to upgrade its MRI system, it frequently takes advantage of the lower cost option of other vendors’ software to assess and manipulate those images.

Two key news items in September made me think about the future direction of hemodynamic support, and that intra-aortic balloon pumps (IABP) will increasingly face a more serious challenge from small percutaneous left ventricular assist devices (pLVAD). This was reinforced when I received a cardiology market analysis from GlobalData agreeing with this assessment.

There were several evident trends on the show floor at RSNA 2012, including interest in software fueled by Stage 1 and 2 meaningful use requirements, new mammography solutions and innovations in imaging hardware.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
More than half of patients treated for claudication or critical limb ischemia (CLI) with stenting of the superficial femoral artery (SFA) had primary patency at five years, says a recent study.
Featuring hovercraft-like C-arm movement and the unique Access Halo, the WorkRite technology on Toshiba America Medical Systems Inc.’s Infinix-i cardiovascular X-ray systems makes interventional procedures easier for clinicians and improves patient care.

Tryton Medical Inc. announced the completion of enrollment in the TRYTON Pivotal U.S. Food and Drug Administration (FDA) IDE trial evaluating the Tryton Side Branch Stent.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Volcano Corporation announced that it has entered into a definitive agreement to acquire Sync-Rx Ltd., a privately-held company based in Israel that develops advanced software applications that optimize and facilitate transcatheter cardiovascular interventions using automated online image processing. It is anticipated the transaction will close within the next 30 days.
December 6, 2012 — Neusoft Medical Systems Co. Ltd., a wholly owned subsidiary of Neusoft Corp., announced this week that its NeuViz 64 multi-slice computed tomography (CT) scanner has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
December 6, 2012 — Daiichi Sankyo Inc. and Eli Lilly and Co. announced results of two retrospective studies comparing rates of readmission for subsequent heart attack and initial hospitalization costs among patients with acute coronary syndromes (ACS) treated with a percutaneous coronary intervention (PCI) and antiplatelet therapy.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Giving clinicians a more complete picture while improving safety during interventional procedures, Toshiba America Medical Systems Inc. introduces Spot Fluoroscopy for its Infinix-i systems. Enabling quicker diagnoses and lower dose, clinicians can observe a target region of anatomy using Spot Fluoro’s live fluoroscopy while viewing the last image hold (LIH) surrounding area.
The U.S. Food and Drug Administration (FDA) granted premarket approval (PMA) for the HeartWare Ventricular Assist System (VAS) designed as a bridge to cardiac transplantation.

As electronic medical records become more sophisticated and healthcare moves to an increasingly paperless system, electrocardiogram (ECG) integration has become a priority for many hospitals. For hospitals that have been using ECG management systems, many are upgrading to newer systems that offer better integration with their ECG systems, interoperability with other software systems, or offer Web-access and smart phone access.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
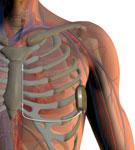
The U.S. Food and Drug Administration (FDA) in October granted Boston Scientific Corp. regulatory approval for its S-ICD System, the world’s first commercially available subcutaneous implantable cardioverter defibrillator (S-ICD). The system sits entirely just below the skin without the need for implantable lead to be placed inside the heart. This offers patients an alternative to transvenous ICDs, which require leads to be placed in the heart itself.
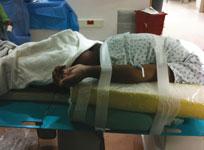
Radial access has been a standard for most patient cases for several years at both the University of Illinois at Chicago (UIC) Medical Center and the Jesse Brown VA Medical Center. The centers are located within two blocks of each other, and the two staffs work very closely. Part of their coordination has been developing training programs and best practices and protocols for radial artery access techniques.
GE Healthcare announced at RSNA 2012 receipt of U.S. Food and Drug Administration (FDA) clearance for its advanced imaging tool, FlightPlan for Liver. Developed to help make intricate liver embolization procedures simpler, FlightPlan for Liver has been commercially available in Europe, Latin America and Asia since 2011, with more than 30 installations in more than 10 countries.

 December 07, 2012
December 07, 2012