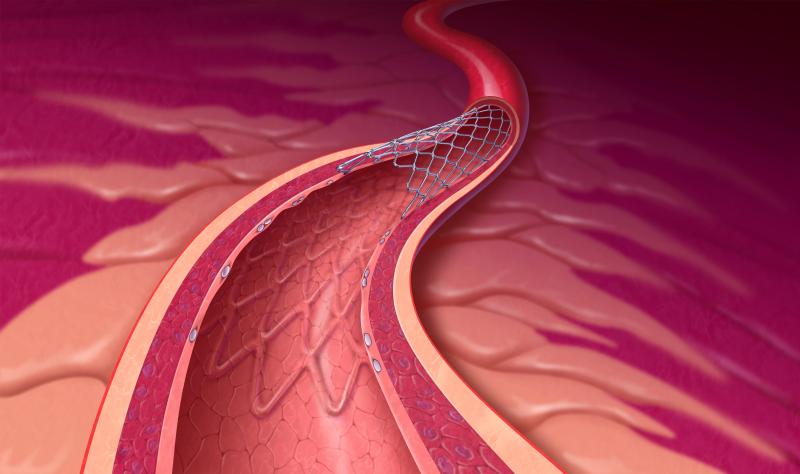
When tests reveal that a patient’s heart artery is blocked and blood flow to the heart is restricted, should the doctor fix it with a stent or stop the test and discuss treatment options with the patient? Recommendations published by the Society for Cardiovascular Angiography and Interventions (SCAI) provide new guidance on when physicians should move forward immediately to open a blocked artery versus when to stop the test for further discussion with the patient or consultation with a heart surgeon.
RamSoft announced its partnership with Digisonics to offer integrated, vendor-neutral cardiac post-processing and structured reporting for all cardiovascular modalities. Digisonics was recently named Best in KLAS for the Cardiology market segment for the fifth consecutive year.
According to Millennium Research Group (MRG), approximately 80 percent of transcatheter aortic valve replacement (TAVR) procedures are being performed on high-risk patients who are ineligible for surgical heart valve replacement. New clinical evidence that supports TAVR in treatment of intermediate and high-risk operable patients has led to expanded reimbursement for a new patient base for the procedure.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Merge Healthcare Inc. announced that Merge Hemo has been named the "Category Leader" in the "2012 Best in KLAS Awards: Software & Services" report for Cardiology Hemodynamics rankings for the second consecutive year.

Some stroke patients may benefit from cerebral angioplasty and stent placement, according to a new study published online in the journal Radiology.
The first patient has been implanted with the Boston Scientific Corporation next generation Ingevity pacing leads in a clinical trial designed to establish the safety, performance and effectiveness of the leads. Pacing leads are insulated wires that connect an implantable pacemaker to the heart for treatment of bradycardia, a condition in which the heart beats too slowly, depriving the body of sufficient oxygen. Pacemakers work in conjunction with leads to sense and stimulate (or pace) the heart, thus maintaining an appropriate heart rate for a given level of physical activity.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Among deployed U.S. service members who died of combat or unintentional injuries between 2001-2011 and underwent autopsies, the prevalence of coronary atherosclerosis was 8.5 percent, with factors associated with a higher prevalence of the disease including older age, lower educational level and prior diagnoses of dyslipidemia, hypertension and obesity, according to a clinical study in the Dec. 26 issue of JAMA.
Pulse Oximetry Screening is a life-saving test that can detect critical congenital heart defects (CCHD) in newborn babies before an infant is discharged from the hospital. The test is easy to perform, however, appropriate interpretation of the results can be challenging. In order to aid health care providers in interpreting the results of the screening, Children's Healthcare of Atlanta has created the Pulse Ox Tool, a ground-breaking app for smart phones that automates the Pulse Oximetry Screening test and improves the accuracy of detecting children with possible CCHD.

The past year in cardiology has continued to see progress and innovation in medicine. As the year comes to an end, the American College of Cardiology (ACC) identified some of the top cardiovascular stories of 2012.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Resuscitation, cell regeneration, a new high blood pressure treatment and developments in devices for treating stroke are among the key scientific findings that make up this year's top cardiovascular and stroke research identified by the American Heart Association and American Stroke Association.
December 28, 2012 — Researchers have discovered that adding lovastatin, a widely used cholesterol-lowering drug, to traditional antimalarial treatment decreases neuroinflammation and protects against cognitive impairment in a mouse model of cerebral malaria.
December 27, 2012 — Cardiosonic Inc., a private developer of technology in the field of renal denervation for the treatment of hypertension, announced it has closed on the first $6.1 million of a planned $16.1 million Series B financing.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
December 27, 2012 — Biotronik announced that the first implant has taken place in the BIOHELIX-I trial in the United States. The BIOHELIX–I trial is set to evaluate the safety and performance of the Pro-Kinetic Energy coronary bare metal stent.
According to Millennium Research Group (MRG), a company focused on global medical technology market intelligence, approximately 80 percent of transcatheter aortic valve replacement (TAVR) procedures are being performed on high-risk patients who are ineligible for surgical heart valve replacement. New clinical evidence that supports TAVR in treatment of intermediate and high-risk operable patients has led to expanded reimbursement for a new patient base for the procedure. The inclusion of these patients will fuel much of the growth of the TAVR market in Europe over the next four years. The market will grow by 60 percent between 2012 and 2016, following 30 percent growth from 2011 to 2012. The European TAVR market will have a total value of $650 million dollars by 2016.
A dozen years ago, controversial clinical trial results caused an international medical society to warn against the use of stents in leg arteries. But recent years have brought significant improvements in stent technology. One-year follow-up results of a worldwide, multicenter trial with 744 patients show that 90 percent of participants had successful procedures that did not require a repeat treatment.

 December 31, 2012
December 31, 2012