January 11, 2013 — Guided Interventions LLC, a startup company developing new, easier to use fractional flow reserve (FFR) a product to help cardiologists better assess the physiological impact of coronary artery blockages, has received a $250,000 investment commitment from nonprofit venture development organization JumpStart Inc.
August 20, 2012 — Zoll Medical Corp., a manufacturer of medical devices and related software solutions, announced that physicians at all of the 17 “Honor Roll” hospitals and all of the 50 “Best Cardiology and Heart Surgery” hospitals designated by U.S. News and World Report for 2012-2013 have prescribed the Zoll LifeVest Wearable Defibrillator for patients.
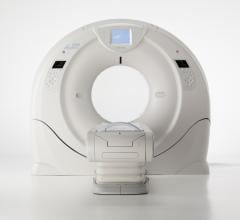
Toshiba has unveiled a 640-slice computed tomography (CT) scanner at the 2012 annual meeting of the Radiological Society of North America (RSNA). The Aquilion One Vision Edition is equipped with a gantry rotation of 0.275 seconds, a 100 kw generator and 320 detector rows (640 unique slices) covering 16 cm in a single rotation, with the industry’s thinnest slices at 500 microns (0.5 mm).
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Zoll’s RescueNet 12-Lead is a free and versatile transmission system that allows healthcare providers to receive and ...
Angioslide announced the closing of $6.3 million financing led by new investor TriVentures. In addition Biostar joined returning investors Viola Partners, Agate and XT Investments to complete the round.
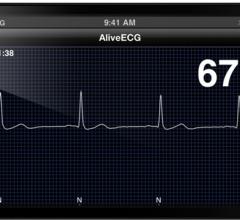
The results of a recent study presented yesterday at the American Heart Association Scientific Sessions 2012 conference in Los Angeles showed that a high-quality single-lead ECG can be quickly and easily recorded using an iPhone with the AliveCor medical device and application to diagnose atrial fibrillation (AF).
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
CardiAQValve Technologies Inc. received $37.3 million in funding in its second round of equity financing. OrbiMed led the round and was joined by Versant Ventures, Advent Life Sciences and existing investors. The funding, which incorporates conversion of a 2011 bridge financing, will be used to further validate the company’s transcatheter mitral valve implantation (TMVI) technology and is expected to carry the company through its feasibility and CE mark clinical trials. Concurrent with the financing, David Bonita, M.D., and Vince Burgess have joined the board of directors representing OrbiMed.
Spectranetics Corp. announced an agreement to purchase the assets of Upstream Peripheral Technologies Ltd., an affiliate of Aran Research Development and Prototypes Ltd. Included in the acquisition are unique technologies that expand Spectranetics' vascular intervention portfolio. A full launch in the United States and Europe will occur in February 2013.
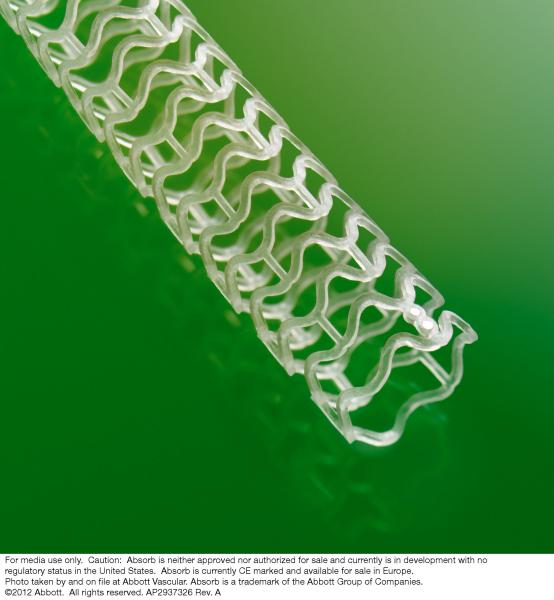
Abbott announced the initiation of the ABSORB III clinical trial in patients in the United States. The randomized controlled trial will enroll about 2,250 patients and compare the performance of Abbott's drug-eluting Absorb boresorbable vascular scaffold (BVS) device to the company's Xience family of drug-eluting stents.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

At RSNA 2012, GE Healthcare announced a first-of-its-kind agreement with the University of Wisconsin (UW) School of Medicine and Public Health for the purpose of providing physicians with more tools to optimize radiation dose, take clinically-useful images, and potentially reduce the frequency of repeat computed tomography (CT) scans.
Biosensors International has announced enrollment of the first patient in LEADERS FREE, a clinical study involving BioFreedom, a polymer-free drug-coated stent (DCS) from Biosensors.
Fovia Medical Inc. and Blackford Analysis Ltd. announced plans to deliver compatible SDKs to the medical advanced visualization market.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Accumetrics Inc. announced that its next generation VerifyNow II System has achieved CE marking for point-of-care measurement of platelet reactivity to antiplatelet agents. This marks the culmination of a series of important milestones in 2012, increasing evidence of the clinical value of platelet reactivity testing and significantly expanding the market opportunities for the VerifyNow System in both surgical and interventional cardiology patient populations.
Biotronik announced that the European Heart Journal has published the clinical results of the ECOST trial (Effectiveness and Cost of ICD Follow-Up Schedule with Telecardiology). The randomized controlled study evaluated the safety and efficacy of Biotronik Home Monitoring compared with standard in-office follow-up visits for patients with implantable cardioverter-defibrillators (ICDs).
January 3, 2013 — Cardiovascular Systems Inc. (CSI) announced it has completed enrollment in its ORBIT II clinical trial, enrolling 443 patients. ORBIT II is evaluating the safety and effectiveness of the company’s orbital atherectomy technology in treating severely calcified coronary arteries.

 January 11, 2013
January 11, 2013