GSI Group Inc., a leading supplier of laser-based solutions, precision motion and optical technologies to global industrial, medical, electronics and scientific markets, announced that it has acquired NDS Surgical Imaging (NDS), a San Jose, Calif.-based global leader in surgical and radiology displays and related peripherals, for $82.5 million in cash, subject to customary closing adjustments.
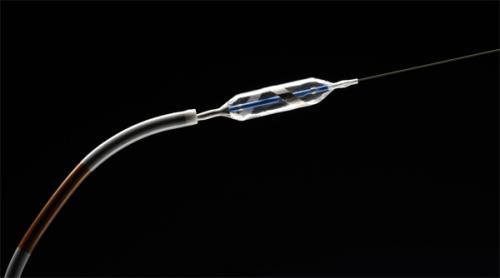
Covidien began the commercial launch of the OneShot Renal Denervation System, an over the wire balloon-based irrigated ablation catheter technology for the treatment of hypertension.

The American Medical Association (AMA) submitted formal comments to the Office of the National Coordinator for Health Information Technology (ONC) on the Health IT Policy Committee’s proposal for Stage 3 of the Medicare/Medicaid meaningful use electronic health record (EHR) program requirements.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Open heart surgery may be a dying art, as new valve replacement techniques offer life-changing treatment without the need for invasive procedures, states a new report by healthcare experts GlobalData.
Lantheus Medical Imaging Inc. has added a low-enriched uranium (LEU) TechneLite (technetium Tc 99m Generator) generator to the its nuclear imaging product portfolio. Lantheus’ LEU TechneLite generator is the first technetium-99m (Tc-99m) generator in the United States that contains molybdenum-99 (Mo-99) produced from at least 95 percent LEU. With greater access to LEU Mo-99 through its supply chain diversification strategy, Lantheus can now move closer to its goal of eventually eliminating Highly Enriched Uranium (HEU)-sourced Mo-99 from its supply chain. Lantheus’ first LEU TechneLite generator was shipped on Jan. 7, 2013.
January 16, 2013 — CardioLogical Solutions, a new cardiovascular device company, announced it has initiated operations. CardioLogical Solutions represents the merger of two independent companies, Emboline and VasoStitch.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
January 16, 2013 — Rex Medical L.P. announced that it has received 510(k) Clearance from the U.S. Food and Drug Administration (FDA) for the Cleaner15 Rotational Thrombectomy System.
January 16, 2013 — Americans' cardiovascular health varies greatly from state to state, according to new research in the Journal of the American Heart Association (JAHA). The study is the first to assess cardiovascular health at the state level.
The U.S. Food and Drug Administration (FDA) has cleared St. Jude Medical’s new version of its Merlin.net Patient Care Network (PCN). The secure, Internet-based remote care system is for patients with implanted implantable cardioverter defibrillators (ICDs), cardiac resynchronization therapy (CRT) devices and pacemakers.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
January 16, 2013 — Medtronic’s CareLink Express service is a remote monitoring system that enables clinicians in healthcare facilities to quickly obtain data regarding the status of Medtronic implanted cardiac devices, facilitating faster treatment decisions.

January 16, 2013 — Just weeks after the Food and Drug Administration (FDA) approved Cook Medical’s Zilver PTX drug-eluting peripheral stent, Riverside Methodist Hospital in Columbus, Ohio, has treated the first patient with the device as part of Cook’s U.S. commercial launch.
Biotronik announced the first U.S. implant of their Pulsar-18 self expanding stent in the BIOFLEX-I IDE clinical trial. Carlos Mena of Yale University Medical Center performed the procedure.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Covidien announced the completion of enrollment in its DEFINITIVE AR (anti-restenosis) study. As the third study in the DEFINITIVE trial series, this randomized pilot is designed to address the challenge of preventing restenosis (re-narrowing of a blood vessel following treatment), a common occurrence in patients with peripheral artery disease (PAD).
According to Millennium Research Group (MRG), the global authority on medical technology market intelligence, the U.S. market for diagnostic imaging equipment servicing will grow slowly to a value of just over $2.78 billion by 2017. Increases in diagnostic imaging system sales and the installed base will be counterbal
McKesson released its Cardiology 13.0 system, which offers a single database solution for cardiac and peripheral catheterization, hemodynamic monitoring, electrophysiology (EP), echocardiography, vascular ultrasound, nuclear cardiology and electrocardiogram (ECG), ECG stress and Holter management. Data entered at any point-of-care flows into the electronic health record (EHR) without the need for potentially cumbersome interfaces or redundant documentation. This latest release is designed to boost clinical efficiencies and reduce operational costs.

 January 17, 2013
January 17, 2013












