October 12, 2012 — Boston Scientific announced it has enrolled the first patient in the REPRISE II clinical trial to evaluate the safety and performance of the Lotus valve system in up to 120 patients with severe aortic valve disease.
October 12, 2012 — Estech, a provider of minimally invasive cardiac ablation devices, last month announced the market release of its Cobra Fusion ablation system.
TyRx Inc. announced that it has received a license from Health Canada to market its AigisRx Antibacterial Envelope. The AigisRx Envelope is specifically designed to stabilize pacemakers and implantable cardioverter defibrillators (ICD) while also releasing antimicrobial agents to help provide protection from microbial colonization of the device during surgical implantation.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
October 11, 2012 — Spectranetics Corp. announced the U.S. market launch of the Tapas catheter at the 2012 Vascular Interventional Advances (VIVA) conference in Las Vegas. Tapas is manufactured by ThermopeutiX Inc. and distributed by Spectranetics.
October 11, 2012 — Edwards Lifesciences Corp. announced it has completed the acquisition of BMEYE B.V., a privately held Dutch company that specializes in the development of noninvasive technology for advanced hemodynamic monitoring.
October 11, 2012 — With this month’s approval of the Standards and Guidelines for Advanced Cardiovascular Sonography by the Commission on Accreditation for Allied Health Education Programs (CAAHEP), experienced cardiovascular sonographers have a new educational pathway for career advancement.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Boston Scientific Corporation has begun the United States and European launch of its Victory guidewire, designed to facilitate crossing of resistant lesions and the placement and exchange of balloon catheters or other interventional devices within the peripheral vasculature. The company expects to launch the product in other international markets later this year and in 2013, subject to regulatory approvals.
New research from the Netherlands suggests tiny microbubbles can be used to more effectively deliver a blood clot busting drug to patients while they are in the ambulance during acute heart attack.
The U.S. Food and Drug Administration (FDA) has granted Boston Scientific Corp. regulatory approval for its S-ICD System, the world's first commercially available subcutaneous implantable cardioverter defibrillator (S-ICD). It sits entirely just below the skin without the need for implantable lead to be placed inside the heart.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
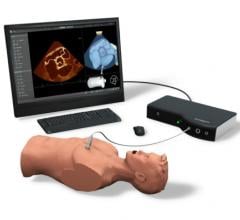
October 8, 2012 — Simbionix USA Corp. will introduce the new advanced U/S (ultrasound) Mentor simulator at the upcoming American College of Emergency Physicians (ACEP) Exhibition in Denver, Colo. This new Simbionix simulator offers realistic hands-on training for diverse ultrasound examinations and interventions.
Following Health Canada approval, Cook Medical has made the Zilver Vena Venous Self-Expanding Stent available to physicians across Canada. Designed to restore blood flow in obstructed iliofemoral veins, Zilver Vena provides physicians with a tool designed specifically for stenting obstructed iliofemoral veins.
October 8, 2012 — St. Jude Medical Inc. has launched MediGuide Technology, the first 3-D navigation system intended for the evaluation of vascular and cardiac anatomy on a recorded fluoroscopic image instead of live fluoroscopy. The use of recorded images allows physicians to reduce the duration of radiation exposure during cardiovascular procedures, especially during long procedures in the electrophysiology (EP) lab.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
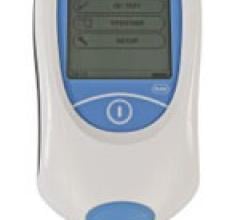
The U.S. Food and Drug Administration (FDA) has granted Clinical Laboratory Improvement Amendments (CLIA)-waived status to the Roche CoaguChek XS Plus system. The point-of-care anticoagulation monitor offers connectivity and data management tools to help healthcare professionals manage PT/INR (blood clotting time) testing. The waiver means that the monitoring technology may now be used in a broader range of clinical settings, such as labs that do not meet the requirements to perform moderate- or high-complexity testing as defined by the CLIA of 1988.
Imagine a place where doctors can tell patients in advance if cancer treatment will work for them, without going through an entire course of chemotherapy.
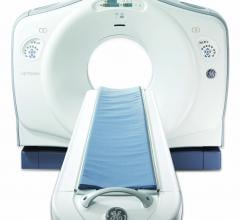
Advocate Health Care, one of the nation's top health systems and the largest integrated health care system in the state of Illinois, and GE Healthcare, a national leader in low dose, high performance imaging, announced a joint effort to help further reduce radiation dose in computed tomography (CT). The goal is to optimize care for patients needing imaging procedures and reduce radiation where possible without adversely impacting image quality. It’s one of the first announcements of the GE Blueprint for low dose, a comprehensive campaign in which GE Healthcare is working alongside leading U.S. health systems to further reduce radiation dose in CT imaging. Leaders from Advocate and GE Healthcare unveiled the Advocate-based GE Blueprint for low dose today during an event at Advocate Lutheran General Hospital.

 October 12, 2012
October 12, 2012