Corgenix Medical Corp. has announced that Atherotech Diagnostics Lab will carry its AspirinWorks Test for determining aspirin effect.
SunTech Medical has updated its line of automated blood pressure (BP) monitoring with the Tango M2 cardiac stress BP monitor. The new product provides accurate BP measurements during exercise and cardiac stress testing where noise and motion often make manual measurements difficult.
Endosense has announced that the results of its EFFICAS I prospective multi-center study have led to the development of guidelines for target and minimum contact force (CF), as well as minimum Force Time Integral (FTI), during the catheter ablation treatment of paroxysmal atrial fibrillation (PAF).
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
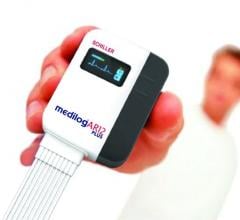
iRhythm Technologies Inc. announced clinical study findings showing that use of the Zio Patch resulted in a change in treatment strategy for nearly one-third of patients with paroxysmal atrial fibrillation (AF). The data, which was published in Pacing and Clinical Electrophysiology (PACE) Journal on March 12, showed that the Zio Patch ambulatory cardiac monitoring system demonstrated improved clinical accuracy and detection of potentially malignant arrhythmias in AF patients compared with a 24-hour Holter monitor in the same patients.

The experimental anti-clotting drug cangrelor solidly outperformed commonly used clopidogrel in a large global trial of patients who underwent coronary stent procedures, according to data from the phase III CHAMPION PHOENIX study presented at the American College of Cardiology’s 62nd Annual Scientific Session.
BioControl Medical has received U.S. Food and Drug Administration (FDA) approval to begin the third and largest phase of INOVATE-HF (INcrease Of VAgal TonE in Heart Failure), a global, multi-center, investigational device exemption (IDE) clinical study of the company’s CardioFit system for heart failure. The approval, which is based on the FDA’s safety review of the first two successful completed phases, allows unconditional study expansion to full enrollment of 650 patients at 80 centers worldwide.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
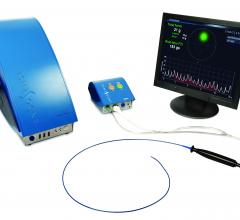
An RTI International-developed prototype catheter that can generate live, streaming 3-D ultrasound images from inside the heart received a Cardiovascular Innovation Award at the 2013 Cardiovascular Research Technologies Annual Symposium.

Cardiac Science is selling its diagnostic cardiology product line to Mortara Instrument Inc. The business consists of the Burdick and Quinton brands and associated products. The transaction does not include MySense, the novel, wearable, single-patient ECG recorder system or the resuscitation business unit, which markets automated external defibrillators (AEDs) worldwide.
Barco announces the launch of its new Eonis clinical display family. Designed with clinical specialists in mind, the Eonis displays combine an innovative, built-in front sensor with Barco’s MediCal QAWeb cloud-based tool to ensure superior, controlled image quality. The Eonis 22-inch model is available in a black and a white version, with the latter offering a fully cleanable front glass panel to prevent infection – a first in the market.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Reva Medical Inc. announced that it has initiated patient enrollment with its ReZolve2 bioresorbable sirolimus-eluting coronary scaffold.
Lantheus Medical Imaging Inc. announced a new supply contract with the Institute for Radioelements (IRE) to receive a supply of molybdenum-99 (Mo-99), the parent isotope of technetium-99m (Tc-99m), for use in its TechneLite (Technetium Tc 99m Generator) generators.
Two new 1.5T MRI scanners from GE Healthcare, the Optima MR360 Advance and Brivo MR355 Inspire, have recently received 510(k) clearance. Redesigned with new enclosures symbolizing GE’s Humanizing MRI strategy, both systems are engineered to address the demand for increased performance and reduced total cost of ownership for the facility, while providing a comfortable experience for the patient.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The American Society of Echocardiography (ASE) has released a list of five interventions whose appropriateness physicians and patients should discuss as part of Choosing Wisely, an initiative of the ABIM Foundation, along with Consumer Reports. Fourth in the list, they ask that patients and their doctors talk about the real need for a stress echocardiogram if they do not present conditions that would warn them of a risk of heart disease.
Rox Medical announced enrollment of the first U.K. patients in the CONTROL-HTN international randomized controlled trial of the Rox Flow procedure for the treatment of resistant hypertension.

This is a roundup of some of the DAIC editor's choice of the most innovative new technologies showcased at ACC 2013, including products in the areas of interventional cardiology, patient monitoring, electrophysiology and echocardiography.


 April 04, 2013
April 04, 2013