The Aspirus Heart & Vascular Institute (AH&VI) services 14 counties in north central Wisconsin and an additional seven counties in the Upper Peninsula of Michigan. The institute receives patients from five hospitals within its system and approximately 10 other hospitals from within this service area. With such a large, mostly rural population — patients come from as far away as 80 miles or more — the organization needed to find a way to more efficiently serve its ST-elevation myocardial infarction (STEMI) and heart valve repair patients.
When cath labs begin performing radial access procedures, both the staff and the operators need to keep an open mind and recognize that it takes time for everyone in the lab to become proficient in doing things in a different way. Learning a new skill can take time and dedicated effort, and as long as one recognizes that, a fledgling transradial program can flourish rather than be abandoned. We offer the following tips for consideration based on our own experience.

There is a growing trend in the use of small, portable extracorporeal membrane oxygenation (ECMO) systems for hemodynamic support. In years past, ECMO systems were a tool of the operating room, primarily for bypass surgery. Older systems required a perfusionist to watch the machine 24 hours a day while it was in operation. Today’s ECMOs use consoles that are much easier to use and do not require a full-time person to monitor. Also, as the technology has been miniaturized, simplified and now uses a percutaneous access system, ECMOs are being used outside the OR, including the cath lab, when a large amount of support is needed that cannot be provided by IABPs or pVADs.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

Since the 1970s, intra-aortic balloon pumps (IABPs) have been the gold standard of minimally invasive hemodynamic support, but more recently developed percutaneous ventricular assist devices (pVAD) are offering an alternative. Clinical data shows pVADs offer more support than IABPs, but their much higher price tag currently restricts their use to niche applications, usually after an IABP fails to deliver enough support.
Live demonstration, transmission or recording of transcatheter aortic valve replacement (TAVR) has been found feasible and safe, even for the typically high-risk patients who undergo the procedure. Results of the VERITAS late-breaking clinical trial, presented at the SCAI 2013 Scientific Sessions, confirmed via comparison of on- and off-camera case studies that recording procedures did not pose any additional risk or complications to patients.
A new method of dynamic, personalized intravenous hydration significantly reduces the percentage of contrast-induced kidney injury from image-guided cardiovascular procedures, according to the results of the POSEIDON clinical trial presented as a late-breaking clinical trial at the SCAI 2013 Scientific Sessions.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
For patients who received stents to restore blood flow through the main arteries supplying blood to the brain, inflating a tiny balloon inside the arteries after implantation of the stent reduced restenosis, or repeat blockages, of the treated arteries, making them less likely to reclose. Modifying the order in which carotid stenting and balloon angioplasty is performed could also alter risk of stroke complications, according to the investigators of the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) sub-study presented at the Society for Cardiovascular Angiography and Interventions (SCAI) 2013 Scientific Sessions.
The impact of pediatric interventional cardiology has never been stronger, nor the need for organized, high-quality training greater, says Frank F. Ing, M.D., FSCAI, who delivered the annual Mullins Lecture at the Society for Cardiovascular Angiography and Interventions (SCAI) 2013 Scientific Sessions. He made the case that advances in technology and growth in the profession mean that now is the time to take training to the next level.

May 13, 2013 — Boston Scientific reports that the four-year follow-up data from the PROTECT AF clinical trial ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Biosense Webster Inc. announced the first patient has been enrolled in the reMARQable clinical study. ReMARQable will assess the safety and efficacy of the use of the nMARQ Pulmonary Vein Isolation System to treat paroxysmal atrial fibrillation. Atrial fibrillation (Afib) is a heart rhythm disorder that affects approximately 20 million people worldwide.
GE Healthcare Innova EPVision 2.0, an application that combines information from electrophysiology (EP) recording and live fluoroscopy, and Dose Blueprint, a comprehensive dose management strategy helping clinicians achieve in particular 50 percent dose reduction on Innova IGS single plane angiography systems compared to previous releases.
The Population Health Research Institute (PHRI) has conducted a further independent analysis of data received from ongoing prospective registries that monitor the performance of the St. Jude Medical Durata and Riata ST Optim implantable cardioverter defibrillator (ICD) leads.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
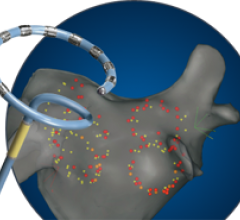
Boston Scientific has received CE Mark approval for the Rhythmia Mapping System, a 3-D mapping and navigation solution for use in cardiac catheter ablations and other electrophysiology (EP) procedures to treat a variety of conditions in which the heart beats abnormally. Some of those conditions include atrial flutter, atrial fibrillation and ventricular tachycardia. Boston Scientific is offering the Rhythmia Mapping System with the company's 64 electrode IntellaMap Orion Mapping Catheter, which has also received CE Mark approval. The combination, part of the 2012 Rhythmia Medical acquisition, is designed to provide electrophysiologists with accurate, high-resolution electro-anatomical maps.
Eizo Nanao Technologies Inc. announced they will unveil the RadiForce RX440, a new 4 MP (2,560 x 1,600 native resolution) color LCD monitor, and the GX540, a 5 MP (2,048 x 2,560 native resolution) monochrome LCD, at the 2012 meeting of the Radiological Society of North America (RSNA).
With an average satisfaction score of 4.5 out of 5 on security, cloud users feel safe. Non-cloud users though remain at bay-particularly with many questions still looming around the future of cloud computing in healthcare. The KLAS report titled Cloud Computing Perception 2013: The Hybrid Cloud in Healthcare looks at the evolution of the cloud in healthcare, provider concerns, as well as vendor performance.

 May 13, 2013
May 13, 2013