CardioKinetix Inc. announced results of a meta-analysis study of the catheter-based Parachute Ventricular Partitioning Device. Six-month clinical results from 91 U.S. and European patients with ischemic heart failure were presented.
InspireMD Inc. announced new six-month results from the MASTER trial demonstrating that the MGuard embolic protection stent (EPS) outperformed bare metal stents and drug-eluting stents in all-cause mortality in ST-segment elevation myocardial infarction (STEMI) patients.
Welch Allyn has released its new FlexiPort EcoCuff blood pressure cuff and EarlySense Vitals surveillance system. Both products enable improvements in patient safety and clinical decision-making to help reduce risk for facilities.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Although not designed for cardiovascular surgery, a new robotic surgical system being developed will likely add to the growing interest of using magnetic resonance imaging (MRI) as a replacement for X-ray angiographic interventional procedural guidance.
Neovasc Inc. reported positive animal data from its Tiara program for the transcatheter treatment of mitral valve disease. Data presented is on long-term implantation of the Tiara mitral replacement valve in an animal model of mitral regurgitation (MR).
Syntermed Inc. has been awarded 510(k) clearance for Emory Cardiac Toolbox version 4.0. "One of the many things that makes Emory Toolbox 4.0 different is SmartReport, the first-ever, cloud-based nuclear cardiology reporting tool using decision support,” said Michael Lee, CEO, Syntermed Inc. The decision support system that powers SmartReport is called Syntermed IDS and will allow diagnosticians to perform faster, more accurate nuclear cardiology reports from single photon emission computed tomography (SPECT) and positron emission tomography (PET) heart scans.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

Intermountain Healthcare, a Utah-based nonprofit system of 22 hospitals and 185 clinics, is compiling the cumulative radiation patients receive from about 220,000 higher-dose procedures and imaging exams each year, starting with exams performed in the last quarter of 2012. That information is now readily available to both physicians and patients.
Unavoidable damage caused to the heart and lungs by radiotherapy treatment of tumors in the chest region can be limited by the administration of an ACE inhibitor, a drug commonly used in the treatment of cardiovascular disease, a group of Dutch researchers have found. [1]
Zoll Medical Corp. has received Shonin approval from the Japanese Ministry of Health, Labour and Welfare (MHLW) to enter the Japanese market with its Intravascular Temperature Management (IVTM) technology — the first intravascular temperature management system to receive Shonin approval in Japan.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
If someone suffers a heart attack while walking down the street and is taken to the hospital quickly, his/her chances of survival are very good. But if someone has a heart attack while already in the hospital for something else, he/she is 10 times more likely to die.
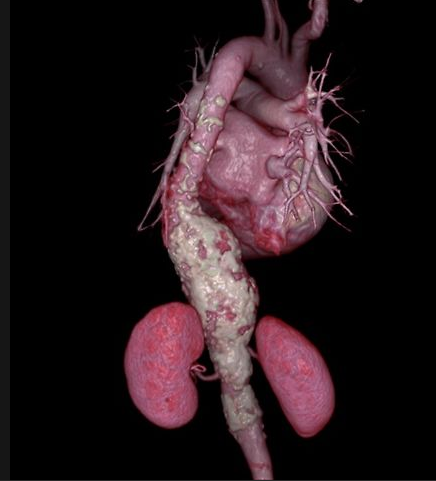
Patients with ruptured abdominal aortic aneurysms (AAA) are more than twice as likely to survive if they have minimally invasive repair than if they have surgery, suggests a 10-year study being.
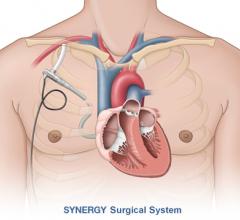
CircuLite Inc. announced that it has received approval from the Federal Agency for Medicines and Health Products in Belgium to commence the CE mark trial of the Synergy IC Circulatory Support System, the first mechanical support system that does not require major surgery. The Synergy IC System is based on the surgical Synergy Circulatory Support System — the world’s smallest commercially available circulatory support pump — which is designed to treat ambulatory chronic heart failure patients (INTERMACS ?4).
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
A device commonly used to treat dangerous heart rhythms may cause more issues for patients than a simpler version of the same device. The implantable cardioverter-defibrillator (ICD) prevents sudden cardiac death by detecting irregularities and delivering an electrical jolt to restart the heart.
Can high blood pressure be safely reduced and controlled by “disconnecting” nerves in the kidneys? That is a question that a new clinical study called Symplicity HTN-3 at University Hospitals (UH) Case Medical Center hopes to answer.
Patients with coronary artery disease who undergo treatment at the University of Maryland Medical Center (UMMC) now can receive long-term therapy based on information found in their genes. As part of a new personalized medicine initiative, the medical center is offering genetic testing to help doctors determine which medication a patient should take after a stenting procedure in order to prevent blood clots that could lead to serious or fatal heart attacks and strokes.


 May 28, 2013
May 28, 2013