Telemed Solutions released its initial product, the TM-12, a PC-based wireless 12-lead electrocardiogram (ECG) system. It embeds a Bluetooth radio into an ECG acquisition device, then streams that ECG data from the patient to a standard Windows computer.
June 3, 2013 — St. Jude Medical Inc. announced the company’s EnligHTN multi-electrode renal denervation system provides a safe, rapid and sustained reduction in blood pressure measurements after one year, as seen in data from the EnligHTN I trial that was presented at EuroPCR 2013 in Paris.

June 3, 2013 — Medtronic Inc. announced it has received CE mark for valve-in-valve (VIV) procedures using the CoreValve and CoreValve Evolut transcatheter aortic valve implantation (TAVI) systems in degenerated bioprosthetic surgical aortic valves.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
June 3, 2013 — OrbusNeich has launched the world's first dual-therapy stent to address the challenges of delayed healing of the coronary artery associated with monotherapy drug-eluting stents (DES), the current standard of care for the treatment of coronary artery disease (CAD).

May 31, 2013 — The American Society of Echocardiography (ASE) released a new expert consensus statement this week focusing on the practice of using ultrasound as a tool for improving patient physical examinations. “Focused Cardiac Ultrasound: Recommendations from the American Society of Echocardiography” will appear in the June issue of the Journal of the American Society of Echocardiography (JASE).
Digisonics will exhibit its Enterprise PACS and structured reporting solutions for cardiology, OB/GYN, radiology and general ultrasound studies at this year’s Society for Imaging Informatics in Medicine (SIIM) Annual Meeting.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

The total U.S. market for PTCA and cutting balloon catheters was valued at $293.5 million in 2012. The PTCA balloon segment accounted for close to 90 percent of the total coronary balloon catheter market, with cutting balloons accounting for the remaining portion. The PTCA balloon catheter market is expected to increase over the next few years and the cutting balloon market is expected to experience a slight decrease.
Welch Allyn has been working with Epic to connect several of its vital signs devices, monitors and electrocardiograph (ECG) units to Epic’s electronic medical records (EMR) system to help eliminate the need for manual data entry by providers, increase efficiency and alleviate the risk of transcription and latency errors. By establishing connectivity between Welch Allyn devices and the EMR, clinicians are able to transfer patient test results directly between diagnostic devices and the EMR — resulting in immediate access to accurate patient data and improved workflow.
Cerebral function is the essence of quality of life. Its preservation throughout medical procedures is a key component to procedural success and patient care. New scientific data presented at the annual EuroPCR Congress in Paris heightens the spreading understanding and recognition that protecting the brain and preserving brain reserve is an important goal of TAVR and other cardiovascular procedures.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Kona Medical announced three and six month results from the WAVE I study, a first-in-man study evaluating the safety and efficacy of Kona Medical’s Surround Sound Renal Denervation System for the treatment of resistant hypertension.
CardioKinetix Inc. announced the transmission of a live satellite feed of a clinical case using the catheter-based Parachute Device. The Parachute procedure was performed by Dr. Martyn Thomas, M.D., chairman of cardiology at St. Thomas’ Hospital in London, England, with assistance from Dr. Hüseyin Ince, professor of medicine, University Hospital Rostock, Germany.

When a power outage occurs, MRI equipment can be damaged and accuracy of the scans effected. To mitigate the effects of power interruptions, healthcare facilities have traditionally relied on the lead-acid batteries of uninterruptible power supplies (UPSs) to provide backup power. However, batteries are notorious for their reliability issues, large footprint and frequent maintenance and replacement.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

Today’s healthcare is changing in many ways and so are the processes, technology and people delivering the extremely complex cardiology procedures and services. There are multiple biomedical and technical advances that are proving to optimize patient care. With declining budgets and reimbursements, healthcare providers are under increasing pressure to develop effective ways of improving efficiency and reducing costs, while maintaining high levels of patient care.

Cardiovascular Systems Inc. (CSI) has presented 30-day results from its ORBIT II study of coronary artery disease. ORBIT II is evaluating the safety and effectiveness of the company’s orbital atherectomy technology in treating patients with severely calcified coronary lesions. This is the first Investigational Device Exemption study to evaluate this problematic subset of patients. CSI completed ORBIT II enrollment of 443 patients at 49 U.S. medical centers in November 2012 and submitted its Premarket Approval application to the U.S. Food and Drug Administration (FDA) on March 15, 2013.
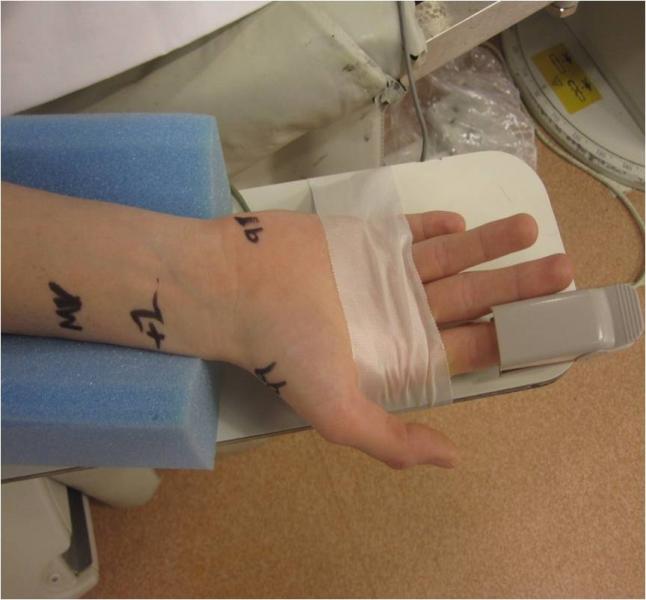
Transradial coronary artery angiography was a major focus at the Society for Cardiovascular Angiography Interventions (SCAI) 2013 Scientific Sessions May 8-11 in Orlando, Fla. The meeting kicked-off with a dedicated symposium covering topics such as access, radiation exposure and complications.


 June 03, 2013
June 03, 2013








