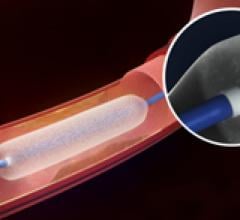
New long-term data from the DIVERGE study, presented at EuroPCR 2013 by Principal Investigator Dr. Stefan Verheye, Antwerp Cardiovascular Centre, ZNA Middelheim Hospital, Belgium, has shown that the use of the Axxess drug-eluting stent (DES) for the treatment of complex coronary bifurcation lesions resulted in low levels of both MACE and VLST over a five-year period. Axxess is now the only dedicated bifurcation stent with a substantial body of supporting data out to five years.
Between June and October, 2013, healthcare providers at hospitals and medical centers across the United States and Canada can experience GE Healthcare’s entire line of Centricity* Imaging technologies when the Centricity Imaging IT Tour visits locations in over 30 major markets.
eHealth Technologies’ eHealthViewer ZF is a zero footprint viewer that enables clinicians, with any secure browser, to instantly access their patients’ medical images, in diagnostic-quality, right from within their patients’ medical record. The latest enhancement to the eHealthViewer ZF is Image Collaboration. Whether through consultation with a remote specialist or grand rounds with the patients’ clinical care team, the eHealthViewer ZF collaboration tool delivers real-time, fluid image review and interaction for healthcare professionals. Using this singular feature, multiple users at different locations, can interactively view and manipulate radiology and cardiology images with full access to the eHealthViewer ZF advanced toolset.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Just a few years ago, integrated positron emission tomography and magnetic resonance (PET/MR) imaging was found only in research institutes, but little by little the technology has expanded into clinical practice. This is especially true for cardiac indications, for which the highly sensitive soft tissue contrast of MR and the functional and metabolic imaging of PET are particularly valuable. New research proves the value of PET/MR compared to PET/computed tomography (CT) in cardiac applications, say researchers at the Society of Nuclear Medicine and Molecular Imaging’s 2013 Annual Meeting.

Direct Flow Medical Inc. announced that it has received approval from the U.S. Food and Drug Administration (FDA) for an Investigational Device Exemption (IDE) to begin the SALUS feasibility trial of the Direct Flow Medical Transcatheter Aortic Heart Valve System. The device encompasses a transcatheter aortic heart valve with a metal-free frame and flexible, low-profile delivery system that virtually eliminates aortic regurgitation. It is designed to improve the long term survivability of patients by resolving the clinical issues associated with current commercial valves.
C. R. Bard Inc. announced the enrollment of the first patient into the Lutonix Below the Knee (BTK) Clinical Trial at The Cardiac and Vascular Institute in Gainesville, Fla. The purpose of this pivotal global, multi-center randomized Investigational Device Exemption (IDE) trial is to compare the safety and effectiveness of the Lutonix 014 Drug Coated PTA Dilatation Catheter to a standard angioplasty balloon catheter for the treatment of critical limb ischemia.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
AngioLight has successfully completed an initial animal study of the AngioLight System at Fu Wai Hospital, a leading cardiovascular center located in Beijing. The study paves the way for AngioLight to go forward in China with full-scale animal and clinical testing of the system, which will begin following the anticipated completion of study protocols, hospital review and required China Food and Drug Administration (CFDA) approvals.
Medtronic Inc. announced new data demonstrating that simultaneously pacing the lower chambers of the heart, or biventricular (BiV) pacing with a cardiac resynchronization therapy (CRT) device, significantly improves heart failure symptoms and quality of life in a subset of heart failure patients.
Led by the launch of the Aplio 500 and Aplio 300 ultrasound systems, Toshiba America Medical Systems Inc. saw a 28 percent U.S. ultrasound business growth in 2012, far exceeding the industry’s 3 percent, according to Klein Biomedical Consultants Inc.’s industry report published in April 2013.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

Extracorporeal membrane oxygenation (ECMO), a procedure traditionally used during cardiac surgeries and in the intensive care unit (ICU) that functions as an artificial replacement for a patient's heart and lungs, has also been used to resuscitate cardiac arrest victims in Japan, Taiwan and South Korea. Now, a novel study of this technique in the United States has been completed by researchers at the Perelman School of Medicine at the University of Pennsylvania, indicating a potential role for this intervention to save patients who are unable to be resuscitated through conventional measures.
GE Healthcare has announced that all of its new computed tomography (CT) products meet the Medical Imaging and Technology Alliance (MITA) smart dose standard that raises the bar for CT dose management.
Boston Scientific Corporation reported interim data from the REDUCE-HTN clinical program, which demonstrated a significant and sustained reduction in the blood pressure of patients treated with the Vessix Renal Denervation System. Data on the first 41 subjects enrolled in the REDUCE-HTN clinical program were presented today at the annual EuroPCR Scientific Program in Paris by Joachim Schofer, M.D., of the Hamburg University Cardiovascular Center.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The American College of Cardiology and the American Heart Association released an expanded clinical practice guideline for the management of patients with heart failure, updating definitions and classifications for heart failure and increasing emphasis on patient-centric outcomes such as quality of life, shared decision making, care coordination, transitions and palliative care.
Data presented at Heart Rhythm 2013 show that Medtronic Inc. Lead Integrity Alert (LIA) software detected pace/sense lead issues in non-Medtronic leads at a greater rate than standard impedance monitoring alone (impedance monitoring measures the electrical continuity of a lead four times per day). The retrospective analysis, which focused on Endotak leads (Boston Scientific) and was presented by Kenneth Ellenbogen, M.D., shows that for every one Endotak pace/sense lead issue detected by impedance monitoring, the LIA software detected four cases that impedance monitoring alone missed. LIA is not currently FDA approved for use with non-Medtronic leads.

The National Institutes of Health (NIH), the nation’s medical research agency and the leading supporter of biomedical research in the world, said all of its clinical programs will be effected by $1.55 billion in NIH budget cuts due to sequestration. On March 1, 2013, as required by statute, President Obama signed an order initiating sequestration. This requires NIH to cut 5 percent, or $1.55 billion, of its fiscal year (FY) 2013 budget. NIH must apply the cut evenly across all programs, projects and activities (PPAs), which are primarily NIH institutes and centers.

 June 11, 2013
June 11, 2013