
The healthcare sector in the United States is facing several reforms, mainly from the implementation of the Patient Protection and Affordable Care Act. However, the results from a survey of 1,710 cath lab clinicians projected a positive outlook for participating clinical professionals with the majority of the respondents reporting higher wages and more than three quarters of the respondents expecting to receive annual salary increases. This indicates that the trend toward higher wages will continue going forward.

One of the exciting new frontiers for interventional cardiology is the use of renal denervation therapy to treat pharmacologically uncontrolled hypertension. It is estimated there are more than 10 million drug-resistant hypertension patients in the United States, so renal denervation (RDN) may offer a massive new market for cath labs to tap into.
Nick Cavros, interventional cardiologist with Cardiovascular Institute of the South (CIS) at Lafayette General Medical Center in Lafayette, La., used a new hand-held mechanical aspiration system to remove blood clots and increase blood flow.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Biotronik has launched its next generation of guide wires. Galeo Pro has a high tensile stainless steel core, increased durability and excellent shape retention. These allow the physician to treat multiple lesions with one wire, thus increasing safety and saving procedure time. This new generation of the classic Galeo guide wire inherits outstanding torque and trackability from previous models.
Epsilon Imaging Inc. has received the CE mark for its EchoInsight visualization and analysis platform with practical strain imaging for improved quality, standardization and workflow in echocardiography interpretation. The CE mark will allow EchoInsight to be sold in the European Union.
To assist providers in managing high-dollar inventory, McKesson has introduced radio frequency identification (RFID) cabinets as part of McKesson Point of Use Supply. The vendor-neutral cabinets now integrate with clinical workflow to better support patient safety while making it easier for providers to monitor inventory and manage expenses, particularly in high-cost specialty areas like cardiology, radiology and operating room (OR) departments.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

GE Healthcare has initiated a Class I recall of all its nuclear imaging systems and called for its systems to not be operated until the vendor can inspect the scanner. The recall follows the death of a patient after a portion of the scanner fell onto the patient during the scan on an Infinia Hawkeye 4 system at a VA medical center facility in the United States.

There is a “new normal” for the global medical device market where financial constraints increasingly impact both treatment and purchasing decision-making. As a result, companies develop products that demonstrate improved clinical outcomes and continue to provide services that raise the quality and efficiency of healthcare delivery in an increasingly resource-constrained environment.
The Boston Scientific Corporation has received U.S. Food and Drug Administration (FDA) clearance, CE mark and Japan PMDA approval for its OptiCross Coronary Imaging Catheter and has launched the device in the United States and Europe. A launch in Japan is planned for later this month. OptiCross, a next generation intravascular ultrasound (IVUS) catheter, offers better deliverability and higher resolution imaging to facilitate complex coronary procedures.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
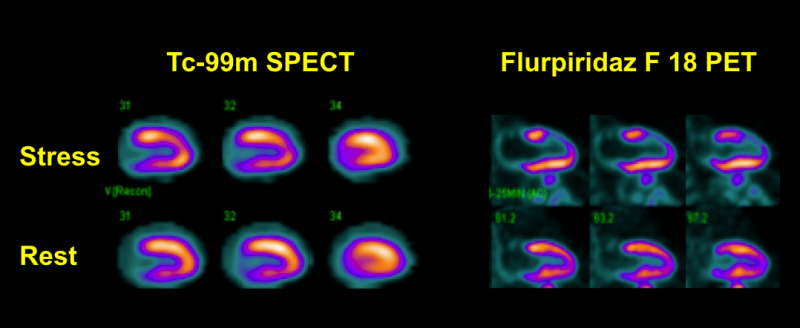
Myocardial perfusion imaging (MPI) with positron emission tomography (PET) has been shown to be superior to single photon emission computed tomography (SPECT). Nevertheless, widespread clinical use of PET MPI has been limited by the currently available PET myocardial perfusion tracers.
Worldwide healthcare cognitive computing markets are poised to achieve continuing growth as the healthcare delivery system responds to new products. Growth is achieved in response to changing technology, better analytics, new information systems that leverage natural language and changing market conditions.
Heart IT has released version 8 of its flagship products: WebPax picture archive and communications system (PACS) and remote viewing system, Universal Viewer.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Merck announced that the New Drug Application (NDA) for its investigational anti-thrombotic medicine, vorapaxar, has been accepted for standard review by the U.S. Food and Drug Administration (FDA).
July 25, 2013 — The 2013 ESC (European Society of Cardiology) Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy, developed in collaboration with the European Heart Rhythm Association (EHRA), have created a new classification system for bradyarrhythmias according to mechanisms rather than aetiology.

Sanford Aberdeen Medical Center in Aberdeen, S.D. became the first hospital to perform a robotic angioplasty for a patient with an acute heart attack, achieving a far better door-to-balloon time than the national standard. Interventional cardiologist Puneet Sharma, performed the percutaneous coronary intervention (PCI) to treat a patient that had experienced a heart attack and presented to the Sanford Aberdeen emergency department. Utilizing the U.S. Food and Drug Administration (FDA)-cleared CorPath System, Sharma was able to perform the robotic-assisted angioplasty procedure and restore blood flow to the patient’s heart within 68 minutes of their arrival.

 July 30, 2013
July 30, 2013












