Todd Dunlap, 62, arrived at Ronald Reagan UCLA Medical Center's emergency room on Aug. 8 suffering from shortness of breath, fatigue and extreme cold. When a computed (CT) scan revealed a 24-inch clot stretching from his legs into his heart, doctors feared the mass could break loose and lodge in his lungs, blocking oxygen and killing him instantly.
OrbusNeich Medical Inc. and Boston Scientific Corp. have settled all stent-related litigation between the companies.
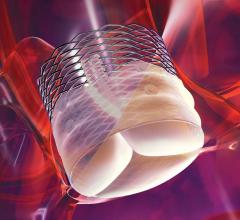
The Mount Sinai Medical Center is participating in the nationwide Absorb III clinical trial testing the performance and potential clinical benefits of a fully dissolvable and temporary drug eluting stent to open heart artery blockages. The randomized trial aims to compare the efficiency and safety of Absorb Bioresorbable Vascular Scaffolds (Absorb BVS) in coronary artery disease patients and compare it to the current standard of care — drug eluting metal stents.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Corindus Vascular Robotics announced the PRECISION Registry, an ongoing study aimed at collecting data on the patterns of use, safety and effectiveness in the delivery and manipulation of percutaneous coronary intervention (PCI) devices. Giora Weisz, associate professor of Medicine at Columbia University Medical Center, will lead the study.
CardiacAssist launched the Protek17 Arterial Cannula, a key component of the TandemHeart temporary circulatory support platform used to rest the heart and circulate blood for patients with severe cardiac dysfunction. Protek17 is designed for improved patient safety and ease of use, with new features including suture wings for secure attachment to the patient and a rubber stop to prevent over-insertion, a common cause of vascular access site bleeding.
Boston Scientific reported favorable six-month results from the first 60 patients enrolled in the REPRISE II clinical trial evaluating the safety and performance of the Lotus Valve System in symptomatic patients with severe aortic stenosis considered at high risk for surgical valve replacement.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
JenaValve Technology Inc. has received CE (Conformite Europeenne) mark approval from European regulators for its transapical TAVI system for the treatment of aortic insufficiency (AI), also known as aortic regurgitation, in which the native aortic valve does not close properly and allows blood to leak back into the left ventricle of the heart.
Decision Resources forecasts that Bayer/Janssen's Xarelto will be the sales-leading therapy among the novel oral anticoagulants in the combined venous thromboembolism (VTE) markets. Xarelto benefits from its first-to-market advantage for VTE treatment/secondary prophylaxis, the removal of the need for bridging with a low-molecular-weight heparin in the VTE treatment/secondary prophylaxis setting and once-daily dosing. Bristol-Myers Squibb/Pfizer's Eliquis will be Xarelto's closest competitor, but its later launch in both the VTE primary prophylaxis and VTE treatment/secondary prophylaxis markets, as well as its twice-daily dosing, will likely limit its sales relative to Xarelto during the 2012 to 2022 forecast period.
Simbionix USA Corp. released the Endovascular Basic Skills training module for the ANGIO Mentor simulator. The Simbionix ANGIO Mentor is a virtual-reality training simulator that provides hands-on practice in a simulated environment for endovascular procedures. An expanding library of modules supports the acquisition and honing of procedural skills to build confidence and proficiency in various endovascular techniques and procedures.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
In a significant milestone toward obtaining additional key regulatory approvals for the Synergy drug eluting stent system, Boston Scientific Corp. has completed enrollment in the EVOLVE II randomized, controlled clinical trial. The EVOLVE II trial is designed to further assess the safety and effectiveness of the Synergy stent system and support U.S. Food and Drug Administration (FDA) and Japanese regulatory approvals for the treatment of atherosclerotic coronary lesions. The Synergy stent uses the market-leading everolimus drug and features an ultra-thin directional polymer coating that is absorbed by the body shortly after drug elution ends at three months.
The new VitalView software from SunTech Medical provides physicians with a data management tool to help efficiently diagnose and treat patients. The new software works with the company’s non-invasive spot check device to reduce the risk for errors in clinical workflows.
The injunction against sales of Medtronic's transcatheter heart valves in Germany — issued in a July 12, 2013, court ruling — has gone into effect, according to Edwards Lifesciences Corp. The District Court of Mannheim ruled that Medtronic is infringing Edwards' Spenser patent for transcatheter heart valve technology and issued an injunction prohibiting the sale of CoreValve and CoreValve Evolut systems in Germany, and ordered a recall of these products. Edwards has now provided the bond required to initiate the injunction.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Heart Imaging Technologies released the WebPAX cardiac echo reporting module.
Oklahoma State University Medical Center, located in Tulsa, Oklahoma, provides high-quality health services to rural and urban Oklahoma. The medical center has a partnership with Oklahoma State University Center for Health Sciences, providing a training ground for healthcare professionals across the region.
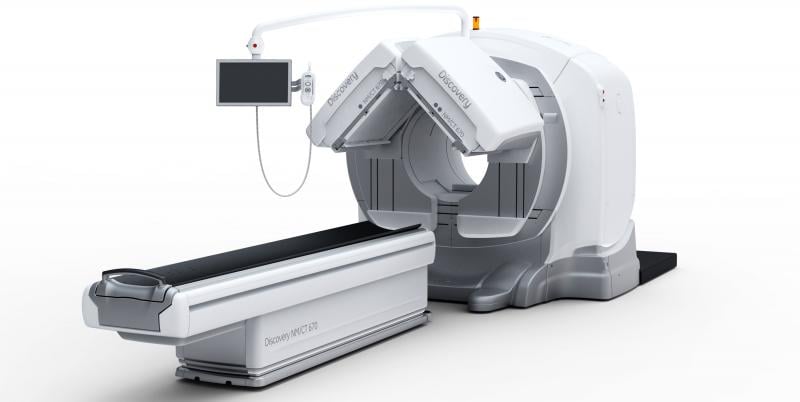
Diagnostic imaging is generally considered safe and noninvasive, so it is extremely unusual for a patient to die from injuries received from a scanner. However, this was the case in early June when a patient was killed because a portion of a SPECT/CT scanner fell during the scan at the James J. Peters VA Medical Center in the Bronx, N.Y.


 September 20, 2013
September 20, 2013