Methodist Le Bonheur Healthcare (MLH) will deploy McKesson’s portfolio of enterprise medical imaging products across its facilities to improve physician collaboration, streamline data management and simplify its imaging archive process with a single point of access.
Rochelle Community Hospital purchased and installed Carestream’s Vue Cardio PACS (picture archive and communications system) that provides fully featured viewing and management for its echo cardiograms and nuclear cardiology exams. This versatile system will also support ECGs and other types of cardiac exams that the critical access hospital may add in the future.
The U.S. Food and Drug Administration (FDA) approved Biotronik’s Ilesto family of implantable cardioverter-defibrillator/cardiac resynchronization therapy defibrillators (ICD/CRT-D devices). Biotronik follows this with the launch of its next generation technology platform Ilesto DX.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The U.S. Food and Drug Administration (FDA) approved the Conformable Gore TAG Thoracic Endoprosthesis for endovascular repair of acute and chronic Type B dissections of the descending thoracic aorta. The Conformable Gore TAG Thoracic Endoprosthesis is designed for multiple thoracic etiologies, and is the only device to receive FDA indications for aneurysm, trauma and dissection. Previously, the only approved treatment options were medical management or open surgical repair.
Expanding its role in the treatment of peripheral artery disease in the United States, Medtronic Inc. announced that the U.S. Food and Drug Administration (FDA) has approved the Complete SE (self-expanding) vascular stent for use in the lower extremities –– specifically, the superficial femoral artery (SFA) and proximal popliteal artery (PPA), which carry blood through the upper legs.
Clinicians face the challenge of effectively imaging larger patients. Recognizing this trend, Esaote’s new eHD Technology improves every element of the imaging chain and increases ultrasound’s ability to image with more clarity at greater depth.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
The Cardiovascular Research Foundation (CRF) announced the late breaking trials and first report investigations that will be presented at the Transcatheter Cardiovascular Therapeutics (TCT) 2013 scientific symposium in late October.
Whole-body magnetic resonance imaging (MRI) may serve as a valuable noninvasive tool for assessing the risk of heart attack and stroke in diabetic patients, according to a new clinical study published online in the journal Radiology.
Sunshine Heart Inc. has launched a website dedicated to providing information to patients suffering from moderate to severe heart failure to learn about its C-Pulse U.S. pivotal trial, COUNTER HF(TM).
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Medtronic Inc. announced clinical trial results showing that heart failure patients treated with its AdaptivCRT feature experienced a nearly 50 percent reduction in atrial fibrillation (AF) risk. Pioneered by Medtronic, the AdaptivCRT technology is a feature on certain cardiac resynchronization therapy-defibrillators (CRT-Ds) that continually adjusts therapy to a patient's natural heart rhythms and minimizes the amount of unnecessary right ventricular (RV) pacing. The results were presented as a late breaking clinical trial at the Heart Failure Society of America's 17th Annual Scientific Meeting.
At RSNA 2013, GE Healthcare will highlight the latest hardware and advanced applications for its Discovery IGS angiography platform. These updates are geared towards addressing the challenges of healthcare providers, support clinical decision making and helping clinicians in refining overall patient care.
Critical Diagnostics announced the clinical study, “Head-to-head comparison of two myocardial fibrosis biomarkers for long-term heart failure risk stratification: ST2 vs. Galectin-3,” recently published online in Journal of the American College of Cardiology (JACC) comparing the company’s cardiac biomarker ST2 to Galectin-3 (Gal-3), a biomarker from BG Medicine, found ST2 to be superior.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Ascendian Healthcare Consulting announced a new service line to assist clients with ensuring the confidentiality, integrity and availability of patient data — the medical imaging client’s privacy, data security and patient safety consulting services.
A study by Cutting Edge Information found that pharmaceutical companies measure cost per patient per month to allow trials of different sizes and durations to be compared easily. This measurement also helps to illustrate the high costs associated with delays.

The U.S. Senate passed legislation Sept. 26 to reauthorize, extend and improve operations of the Federal Helium Reserve in Texas, which will guarantee a stable supply of the gas used for cooling magnetic resonance imaging (MRI) systems.

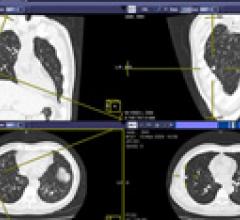
 October 02, 2013
October 02, 2013