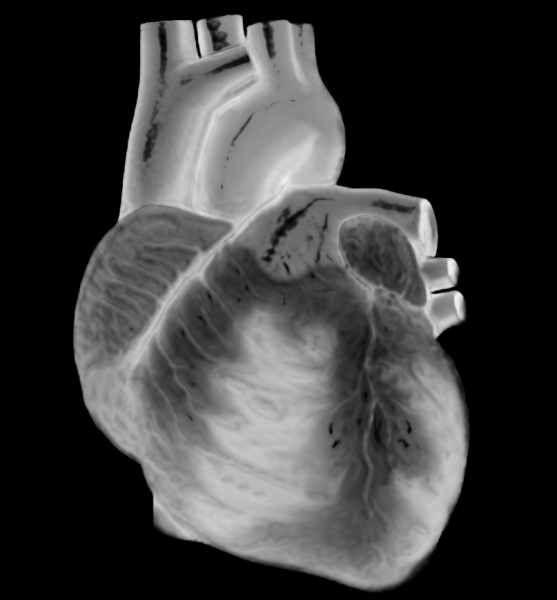
Clinical trials for a promising new class of cholesterol-lowering medications, a long-awaited repair of the Medicare formula for paying physicians, the Affordable Care Act, new technology and the implementation of new prevention guidelines are among the top developments in cardiology anticipated for 2014.

It was not until we began planning that we realized just how involved TAVR programs are — they require much more work than we originally anticipated. At Terrebonne General Medical Center (TGMC), our TAVR program started as part of a research project in our structural heart department. It was an opportunity to offer advanced patient care and determine the effectiveness of TAVR on patients experiencing severe aortic stenosis, who were deemed inoperable by two cardiac surgeons.
Intact Vascular Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, announced the start of enrollment in the Tack Optimized Balloon Angioplasty Below the Knee (TOBA-BTK) study.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Mobile Aspects announced that it has been awarded the patent for its iRISupply radiofrequency identification (RFID) system that supports the storage and management of high-value medical devices and surgical products used in specialty areas of a hospital.
Atrial fibrillation (AF), long considered the most common condition leading to an irregular heartbeat, is a growing and serious global health problem, according to the first study ever to estimate the condition’s worldwide prevalence, death rates and societal costs.
Careful documentation of patient selection and procedure appropriateness are critical — yet underutilized — steps in ensuring high-quality clinical care for percutaneous coronary intervention (PCI).
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
AliveCor announced Android compatibility for its AliveInsights analysis service, which launched for iOS (iPhone) users of the AliveCor Heart Monitor Nov. 18, 2013.
Following public comment received in the fall of 2013, The Joint Commission has released new accreditation standards for diagnostic imaging services. Nearly all of these new standard Elements of Performance (EP) are slated to become effective in a half-year.
Johns Hopkins Hospital surgeons say skipping one commonly taken step during a routine procedure to insert a wire mesh stent into a partially blocked carotid artery appears to prevent patients from developing dangerously low blood pressure, an extremely slow heart rate or even a stroke or heart attack.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Ultrasound-stimulated microbubbles have been showing promise in recent years as a non-invasive method of breaking up dangerous blood clots.
Toshiba America Medical Systems Inc. introduced enhancements to its Vantage Titan magnetic resonance (MR) product line.

The American College of Radiology (ACR) commends the House Committee on Ways and Means and the United States Senate Committee on Finance for passing bicameral, bipartisan legislation to replace the flawed sustainable growth rate (SGR) physician payment formula.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
A new study by researchers at the University of California, Los Angeles (UCLA) Fielding School of Public Health and McGill University in Montréal reveals that the United States health care system ranks 22nd out of 27 high-income nations when analyzed for its efficiency of turning dollars spent into extending lives.

The American College of Cardiology (ACC), along with nine specialty and subspecialty societies, released Appropriate Use Criteria (AUC) for tests used in the diagnosis and/or evaluation of stable ischemic heart disease.
CSL Behring announced that Kcentra (Prothrombin Complex Concentrate [Human]) has received U.S. Food and Drug Administration (FDA) approval for an expanded indication — urgent reversal of acquired coagulation factor deficiency induced by vitamin K antagonist (VKA) (warfarin) therapy in adult patients needing an urgent surgery or other invasive procedure.

 December 24, 2013
December 24, 2013










