Argon Medical Devices Inc. received clearance from the U.S. Food and Drug Administration (FDA) to begin marketing the OptionElite retrievable inferior vena cava (IVC) filter with an over-the-wire delivery technique.
An international research team, led by Icahn School of Medicine at Mount Sinai investigators, designed and tested a high-density lipoprotein (HDL) nanoparticle loaded with a statin drug. In mouse studies, they show HDL nanotherapy is capable of directly targeting and lowering dangerous inflammation in blood vessels.
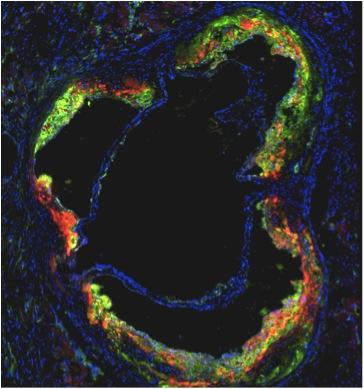
An international research team at Icahn School of Medicine at Mount Sinai is testing a sugar-based tracer contrast agent to be used with positron emission tomography (PET) imaging to help identify dangerous inflammation and high-risk vulnerable atherosclerotic plaque inside vessel walls that causes acute heart attacks and strokes.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
An implant of the ReZolve2 bioresorbable scaffold was transmitted live via satellite at the Transcatheter Cardiovascular Therapeutics (TCT) 2013 conference in San Francisco.
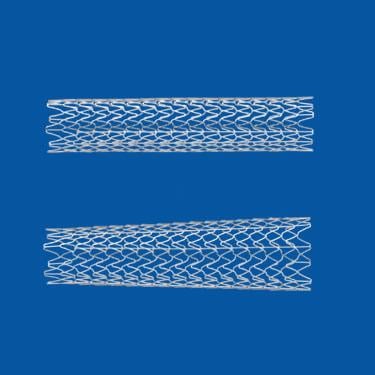
The three most common systems to place stents in blocked carotid arteries of the neck have similarly low rates of complication and death among U.S. patients, according to a study published by JACC: Cardiovascular Interventions.
Research published in the American Journal of Managed Care indicated using telemedicine to deliver stroke care, or telestroke, appears to be cost-effective for society. Telestroke robots allow stroke patients in facilities with no neurology specialists to receive real-time consultation from neurologists in other areas via computer.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
The U.S. Food and Drug Administration (FDA) gave market clearance to Irvine Biomedical Inc., a St. Jude Medical company, for its Therapy Cool Flex Ablation Catheter and IBI 1500T9-CP v.1.7 Cardiac Ablation Generator.
The U.S. Food and Drug Administration (FDA)’s Cardiovascular and Renal Drugs Advisory Committee recommended approval of vorapaxar, Merck’s investigational antiplatelet medicine for the reduction of atherothrombotic events, when added to standard of care in patients with a history of heart attack and no history of stroke or transient ischemic attack.
The U.S. Food and Drug Administration's (FDA) Cardiovascular and Renal Drugs Advisory Committee voted against the approval of the use of oral anticoagulant Xarelto (rivaroxaban) to reduce the risk of thrombotic cardiovascular events in patients with Acute Coronary Syndrome (ACS) in combination with standard antiplatelet therapy. Janssen Research & Development LLC sought approval of rivaroxaban at a proposed dose of 2.5 mg twice daily (BID) for a 90-day treatment duration.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The first commercial implant of Elixir Medical’s CE Mark-approved DESolve Novolimus Eluting Coronary Scaffold was performed in Germany. Elixir’s fully bioresorbable DESolve scaffold for coronary artery disease restores blood flow to the heart like metallic stents and then dissolves, resulting in a functional artery free of a permanent implant.
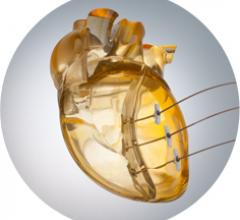
BioVentrix’s Revivent-TC Ventricular Enhancement System was implanted in a man via Less Invasive Ventricular Enhancement (LIVE) in Prague, Czech Republic. The procedure, which is used to reshape and reduce the left ventricle, was performed on a 64-year-old man suffering from ischemic heart failure.
BeneChill Inc. received the 2014 Frost & Sullivan Award for Technology Innovation Leadership for its RhinoChill intranasal cooling system. BeneChill's RhinoChill is a nasopharyngeal device that can induce and sustain therapeutic hypothermia during active resuscitation after cardiac arrest. The system is noninvasive, time critical and can be used by nonmedical personnel to prevent brain damage associated with cardiac arrest.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

As the world becomes more wired and mobile devices offer instant access to email, apps and the Web, it is only natural this technology is migrating into the healthcare sector, where physicians are seeking workflows similar to what they are familiar with when using their devices during free time. In addition to anywhere access to patient records, mobile devices can enable new functionality, such as critical results communication, mobile radiology image viewing for referring physician access, electrocardiogram (ECG) waveform access for immediate ST segment elevation myocardial infarction (STEMI) or other emergency assessment, and the ability to view completed reports on the go.
Computed Tomography (CT) procedure rates have declined by an average annual 5.5 percent in the United States over the past two years, diverging from a previous decade-long growth trend, according to a survey conducted by IMV Medical Information Division.

The Cardiovascular Research Foundation’s Transcatheter Cardiovascular Therapeutics (TCT) 2013 offers many new insights about the latest cardiovascular technologies and treatment techniques. These are my choices for the most innovative new or futuristic technologies highlighted on the show floor or in sessions at TCT 2013.


 January 23, 2014
January 23, 2014