I have watched a trend in medical technology grow from a small ripple a couple years ago into what I expect will be a tidal wave in 2014 due to growing concern over patient radiation dose levels from medical imaging. Use of radiation dose monitoring software came to the forefront when California, followed by Texas, created laws requiring medical facilities to record the amount of exposure patients receive from things like computed tomography (of which cardiac exams are among the highest dose levels used) and angiography. Adoption of this software will be further accelerated by new Joint Commission standards released in December 2013, which starting in mid-2014 requires the use of radiation dose monitoring software as part of its accreditation.
St. Jude Medical Inc. announced the first enrollments in the company’s LEADLESS Pacemaker Observational Study evaluating the Nanostim leadless pacing technology. The Nanostim pacemaker received CE marking in 2013, and post-approval implants have occurred in the United Kingdom, Germany, Italy, Czech Republic, France, Spain and the Netherlands.
Lumedx Corp. is a provider of vendor-neutral cardiovascular imaging and information systems (CVIS). It announced it has contracted to provide Phoebe Putney Memorial Hospital with a comprehensive CVIS that will integrate patient data and images across five different sites. The Lumedx CVIS will consolidate data, improve physician access to patient information, streamline clinical workflows, and support outcomes analysis and registry reporting for the Phoebe Heart and Vascular Center.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Thoratec Corp. initiated a voluntary worldwide Medical Device Correction in order to update its labeling and training materials for the HeartMate II LVAS Pocket System Controller. The following information is provided as a reinforcement of the initial release.
Patients with coronary artery disease who received an Endeavor or Resolute drug-eluting stent from Medtronic Inc. and subsequently interrupted their dual antiplatelet therapy (DAPT) earlier than current guidelines recommend experienced no increased risk of stent thrombosis at one year or more of follow up. Both devices elute the drug zotarolimus to reduce the risk of restenosis. This is according to findings from several studies presented at the 25th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium.
The increasing number of percutaneous coronary interventions (PCIs) being performed at low-volume centers without on-site cardiac surgery backup has driven the need for new safety and quality protocols. This is according to an expert consensus document written by a committee representing the Society for Cardiovascular Angiography and Interventions (SCAI), the American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA). The document outlines steps hospitals can take to provide the safest possible environment for PCI when the facility does not provide cardiac surgery as a backup should complications occur.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Generating new cardiac muscle from human embryonic stem cells (hESCs) and/or induced pluripotent stem cells (iPSC) could fulfill the demand for therapeutic applications and drug testing. The production of a similar population of these cells remains a major limitation, but in a study just published in Stem Cells Translational Medicine, researchers now believe they have found a way to do this.
Volumes are high at the Bryan Medical Center/Bryan Heart Hospital, located in Lincoln, Neb., resulting in large amounts of data. Managing and monitoring all this data is critical, so the center turned to Lumedx Analytics performance management software. The new solution, staff says, has turned out to be both versatile and powerful.
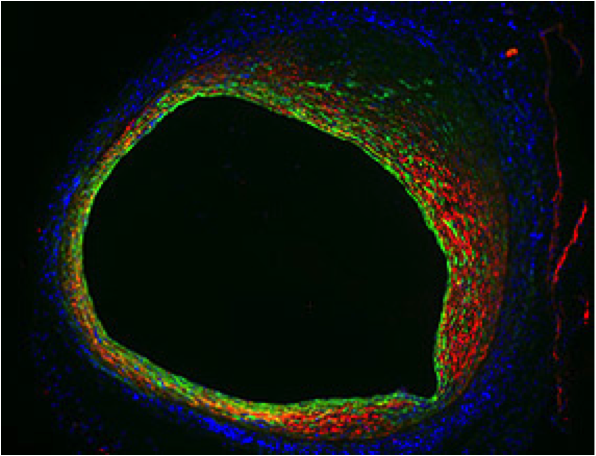
National Institutes of Health researchers have identified a biological pathway that contributes to the high rate of vein graft failure following bypass surgery. Using mouse models of bypass surgery, they showed that excess signaling via the transforming growth factor beta (TGF-Beta) family causes the inner walls of the vein become too thick. This slows down or sometimes even blocks the blood flow that the graft was intended to restore. Inhibition of the TGF-B signaling pathway reduced overgrowth in the grafted veins.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Boston Scientific Corp. received CE marking for the Rebel Platinum Chromium Coronary Stent System, the company's latest generation bare metal stent for the treatment of coronary artery disease (CAD).
Janssen Research & Development LLC (Janssen) announced the U.S. Food and Drug Administration (FDA) issued complete response letters (CRLs) regarding supplemental New Drug Applications (sNDAs) for the use of rivaroxaban, an oral anticoagulant, to reduce the risk of secondary cardiovascular events — defined as heart attack, stroke or death — in patients with acute coronary syndrome (ACS) and to reduce the risk of stent thrombosis in the same population, in combination with standard antiplatelet therapy.
Privately held On-X Life Technologies Inc. (On-X LTI) launched its European marketing campaign for the On-X Plus 1.5 Aortic Heart Valve in concert with its Great Britain distributor Vascutek Corp. at the Annual Meeting & Cardiothoracic Forum of the Society for Cardiothoracic Surgery in Great Britain & Ireland, March 10-12, Edinburgh, Scotland.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Micell Technologies Inc. announced that imaging and clinical results from the DESSOLVE I and DESSOLVE II trials of its MiStent Sirolimus Eluting Absorbable Polymer Coronary Stent System (MiStent SES) were presented at the 25th Annual Transcatheter Cardiovascular Therapeutics (TCT) Conference held in San Francisco, Oct. 27 to Nov. 1, 2013.
Houston Healthcare is putting patient safety first by offering low dose computed tomography (CT) exams with the industry’s best solutions and a comprehensive dose management approach from Toshiba America Medical Systems Inc.
iRhythm Technologies Inc., a healthcare information services company, announced that new study data support the use of its ZIO Service to help identify underlying cardiac arrhythmias in patients who have had a stroke or transient ischemic attack (TIA).

 March 18, 2014
March 18, 2014