Wolters Kluwer Health announced that Kettering Health Network has selected ProVation Order Sets, powered by UpToDate Decision Support, as its clinical content management system.
Among the collection of aspiring medical isotope production companies looking to build facilities in the United States, Coquí RadioPharmaceuticals Corp. is he only one using a design and production method already proven in the commercial markets. Coquí is planning on constructing and operating a dedicated medical isotope production facility.

June 5, 2014 — Fairview Health Services, Minneapolis, recently became the first U.S. healthcare facility to install the Symbia Intevo xSPECT system from Siemens Healthcare. The first ever xSPECT system, the Symbia Intevo fully integrates single-photon emission computed tomography (SPECT) and CT during image reconstruction, combining SPECT’s high sensitivity with the high specificity of CT and completely integrating data from the two modalities to generate high resolution and, for the first time, quantitative images.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
June 5, 2014 — Mercator MedSystems is in the midst of its prospective, 300-patient, 30-site DANCE (Dexamethasone to the Adventitia to eNhance Clinical Efficacy) clinical trial to study a new therapeutic approach to treating peripheral artery disease (PAD). The trial enrolled its first patient in November 2013, and incorporates the use of Mercator Med Systems’ U.S. Food and Drug Administration (FDA)-cleared Micro-Infusion drug delivery system.
June 5, 2014 — Lumedx Corp. and Scientific Software Solutions Inc. (SSS), maker of PedCath congenital catheterization reporting software, announced they are partnering to create an innovative solution for congenital cardiac data and image management.

June 5, 2014 — The president of the American College of Cardiology (ACC), Patrick O’Gara, M.D., recently explored what is causing the looming interventional cardiologist shortage, how it could threaten the quality of patient care and what can be done about it in the annual Hildner Lecture at the 2014 Society for Cardiovascular Angiography and Interventions (SCAI) scientific sessions.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
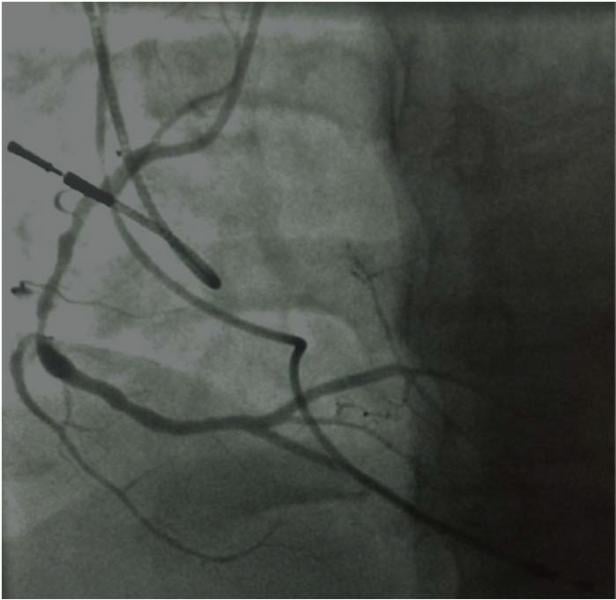
June 5, 2014 — New measures of the severity of coronary artery blockages do not provide enough accuracy to guide treatment decisions, according to the results of the VERIFY-2 study presented as a late-breaking clinical trial at the Society for Cardiovascular Angiography and Interventions (SCAI) 2014 scientific sessions in Las Vegas.
Health Media Network (HMN) and the American College of Cardiology (ACC) have announced a strategic partnership that will significantly increase the size of HMN's cardiology network while broadening the distribution of ACC's CardioSmart initiative.
Cook Medical is engaged in two clinical studies that will provide additional data on the safety and effectiveness of inferior vena cava (IVC) filters.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The Accreditation for Cardiovascular Excellence (ACE) Board of Directors has approved a new set of Performance Metrics for Cath PCI Accreditation.
Biotronik has expanded its line of reinforced introducer sheaths.
The U.S. Food and Drug Administration (FDA) recently cleared BB Medical Surgical’s Thixo-Gel, which is sprayed on to the patient to enable quicker and easier ultrasound scans.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Medtronic Inc. announced CE mark and launch of the NC Euphora noncompliant balloon dilatation catheter.
Strokes kill nearly 130,000 Americans every year, according to the Centers for Disease Control and Prevention. Because approximately 30 percent of strokes are caused by blockages in the carotid arteries — the blood vessels that supply blood to the brain— successful treatment of carotid disease could save thousands of them, according to the Society for Vascular Surgery, members of which will discuss and debate treatment options for carotid disease at the 2014 Vascular Annual Meeting, taking place June 4-7 at the Hynes Convention Center in Boston.
June 3, 2014 — Edwards Lifesciences Corp. received CE mark for the advanced Edwards Intuity Elite valve system. The next-generation, rapid deployment system facilitates smaller incisions in surgical aortic valve replacement (AVR) procedures, and is built upon extensive evidence supporting the durability of the Carpentier-Edwards Perimount heart valve design.

 June 06, 2014
June 06, 2014










