Interventional thought leaders at an American College of Cardiology (ACC) meeting shared their predictions about the cutting-edge technologies emerging today that will become commonplace in the cath lab of the future.
C. R. Bard Inc. announced that the U.S. Food and Drug Administration’s (FDA) Circulatory System Devices Advisory Panel provided a unanimous favorable recommendation to FDA for use of the Lutonix Drug Coated Balloon PTA Catheter (DCB) in the United States.
New appropriate use expert consensus documents developed by the Society for Cardiovascular Angiography and Interventions (SCAI) provide guidance on treating the most common form of peripheral artery disease (PAD), namely blockages that affect arteries above the knee.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
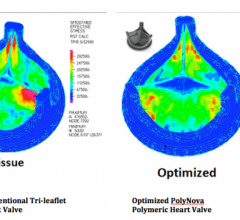
June 16, 2014 — PolyNova, a startup company that has grown out of an inter-institutional collaboration between the University of Arizona (UA) and Stony Brook University, announced it is developing a novel polymeric prosthetic heart valve. Sarver Heart Center cardiologist Marvin J. Slepian, M.D., professor of medicine and biomedical engineering at the UA, is the founding CEO of the new venture. PolyNova entered an exclusive option to the patent rights jointly held by the two universities earlier this spring.
June 16, 2014 — Carestream’s Smart Link helps ensure optimal system performance and uptime by continuously monitoring operations of its healthcare IT and digital imaging systems at healthcare providers across the globe. Smart Link is provided as part of Carestream’s customer service maintenance agreements.
June 16, 2014 — Boston Scientific Corp. has initiated the RESPOND post-market registry to assess real-world performance of the Lotus valve system. The RESPOND registry will collect data on clinical outcomes and device performance in 1,000 patients implanted at 50 centers around the world.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

June 16, 2014 — Children with heart disease are exposed to low levels of radiation during X-rays, which do not significantly raise their lifetime cancer risk. However, children who undergo repeated complex imaging tests that deliver higher doses of radiation may have a slightly increased lifetime risk of cancer, according to researchers at Duke Medicine. The findings, published June 9 in the American Heart Association (AHA) journal Circulation, represent the largest study of cumulative radiation doses in children with heart disease and associated predictions of lifetime cancer risk.

Medtronic announced it is purchasing Covidien in a cash-and-stock deal for $42.9 billion. Once the transaction is completed, Medtronic will have significantly advanced its position as a premier international medical technology and services company.
Cardinal Health announced the 2 millionth shipment of the Mynx Vascular Closure Device (VCD).
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
MedAptus announced First Coast Cardiovascular Institute (FCCI) is live on the company's Professional Charge Capture (Pro) application and is already experiencing significantly reduced charge lag just weeks after installation.
GE Healthcare introduced the Discovery NM/CT 670 Pro at the Society of Nuclear Medicine and Molecular Imaging Annual Meeting (SNMMI 2014). Quantitative accuracy in nuclear medicine is enabled by Q.Metrix and Q.AC, GE Healthcare's newest software innovations in nuclear medicine.

June 12, 2014 — Three-dimensional imaging known as 3-D quantitative coronary angiography (3D-QCA) accurately identifies hard-to-see coronary artery lesions that merit further evaluation, according to the IQ-CATEGORIZE Lesions study presented as a late-breaking clinical trial at the Society for Cardiovascular Angiography and Interventions (SCAI) 2014 scientific sessions in Las Vegas.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The U.S. Food and Drug Administration (FDA) today expanded the indicated use for the CoreValve self-expanding transcatheter aortic valve system for patients with severe aortic stenosis who are at high risk for surgery. CoreValve originally gained FDA clearance in January for patients at extreme risk for surgical valve replacement. This new approval is based on groundbreaking data that showed clinical outcomes at one year with the CoreValve system were superior to the current gold standard of open-heart surgery.
June 12, 2014 — Philips Healthcare recently introduced Vereos PET/CT, the first digital PET/CT (positron emission tomography/computed tomography) scanner, at the 2014 annual meeting of the Society of Nuclear Medicine and Molecular Imaging (SNMMI) in St. Louis. In addition to Vereos, Philips showcased a selection of molecular imaging solutions designed to deliver high image quality, critical clinical information and greater connectivity.
Ischemic heart disease, a narrowing of the arteries supplying blood to the heart, is a leading cause of death throughout the world. A hybrid molecular imaging technique called positron emission tomography and magnetic resonance (PET/MR) imaging, which tells doctors vital information about cardiac and arterial function, has been found to be an effective molecular imaging tool for detecting coronary artery disease (CAD), say researchers at the Society of Nuclear Medicine and Molecular Imaging’s 2014 Annual Meeting (SNMMI).

 June 17, 2014
June 17, 2014