September 5, 2014 — The number of uninsured is expected to decline by nearly half from 45 million in 2012 to 23 million by 2023 as a result of the coverage expansions associated with the Affordable Care Act (ACA), according to a report from the Centers for Medicare & Medicaid Services (CMS) Office of the Actuary. The report is published in Health Affairs.
September 5, 2014 — CardiacAssist announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its new Protek Duo veno?venous cannula. The Protek Duo is intended for use as a single cannula for both venous drainage and reinfusion of blood via an internal jugular vein during extracorporeal life support (ECLS) procedures.
A new analysis of clinical trial participation in the largest ongoing observational study of U.S. heart attack patients has found participants are not representative of the larger patient base, according to a study led by Women’s College Hospital cardiologist Jay Udell.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
AirStrip, a provider of mobile healthcare applications, has raised $25 million in a strategic funding round led by the Gary & Mary West Health Investment Fund, Sequoia Capital and Wellcome Trust that includes some of the most influential leaders in healthcare.

September 4, 2014 — Medtronic announced CE mark for the 23 mm CoreValve Evolut R system for transcatheter aortic valve implantation (TAVI). The novel self-expanding valve and 14 French equivalent delivery system offers new capabilities that advance valve performance and deliverability during the procedure, while providing the option to recapture and reposition the valve during deployment phase if needed.
September 4, 2014 — St. Jude Medical announced a new multicenter clinical trial has found that using the company’s fractional flow reserve (FFR) technology changed the course of treatment for more than one-fifth of patients suffering non-ST segment elevation myocardial infarction (NSTEMI) heart attacks.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
The Cardiovascular Cell Therapy Research Network (CCTRN), has selected Cytori Therapeutics, Inc. to supply adipose-derived regenerative cells (ADRCs) for a clinical trial aimed at evaluating the safety and feasibility of treating patients with Left Ventricular Assist Devices (LVADs).
For the first time in the United States, doctors at Henry Ford Hospital used a minimally invasive procedure to replace a failing, hard-to-reach heart valve with a new one – and placed it just outside the heart.
Angiographic imaging system vendors have developed several new technologies to address emerging cath lab trends, including the need to reduce radiation dose, improve image quality and enable advanced procedural image guidance. All three of these points have become increasingly important as more complex procedures are attempted in interventional cardiology cath labs and hybrid ORs. These procedures include transcatheter aortic valve replacement (TAVR), mitral clip valve repairs, left atrial appendage (LAA) occlusions, atrial and ventricular septal defect closures, and new interventions for both electrophysiology (EP) and heart failure.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
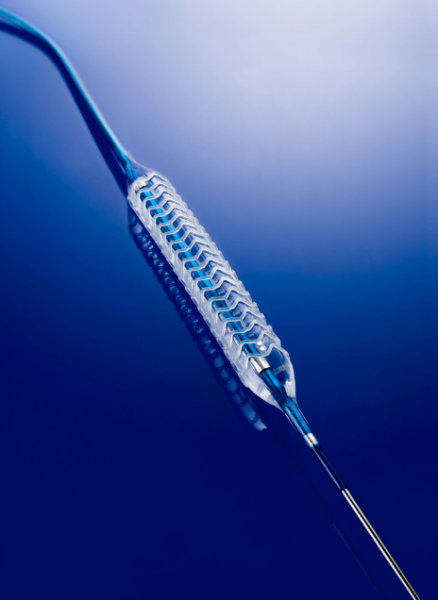
A key prediction for interventional cardiology coming out of recent Transcatheter Cardiovascular Therapeutics (TCT) and American College of Cardiology (ACC) meetings is that bioresorbable stents will eventually replace conventional metallic stents in the coming years. Experts say dissolving stents have their drawbacks compared to metallic stents, but as the technology continues to advance, these issues may be resolved. Even if they are not, experts say growing clinical data shows the benefits of bioresorbable stents may outweigh any drawbacks.
As technology continues to advance for all diagnostic imaging modalities, it sometimes reminds me of a race between vendors to build a better mouse trap. The main issue between cardiac echo, computed tomography (CT), magnetic resonance imaging (MRI), nuclear (SPECT and PET) and invasive angiography is that each has its strengths and weaknesses.
In patients who experienced an acute coronary syndrome (ACS) event, use of the drug darapladib to inhibit the enzyme lipoprotein-associated phospholipase A2 did not reduce the risk of recurrent major coronary events.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
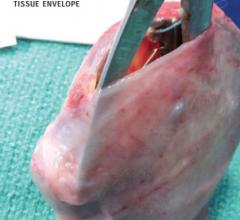
CorMatrix Cardiovascular announced that it has received U.S. Food and Drug Administration (FDA) clearance to market the CorMatrix CanGaroo ECM Envelope for use with cardiac implantable electronic devices (CIED’s) including pacemakers and implantable cardioverter defibrillators (ICD’s).
September 3, 2014 — Jobst Vascular Institute is conducting pioneering research that looks at using stents to treat blocked veins. The Cook Medical VIVO clinical research study aims to determine the safety and effectiveness of the Zilver vena venous self-expanding stent in the treatment of vein obstructions.
September 3, 2014 — Teleflex announced a newly published clinical study demonstrating the accuracy of the Arrow VPS G4 vascular positioning system, with placements of peripherally inserted central catheters (PICCs), can eliminate the use of confirmatory chest X-rays.


 September 05, 2014
September 05, 2014