
According to a new study, transcatheter aortic valve replacement (TAVR) provided meaningful clinical benefits relative to surgical aortic valve replacement (SAVR) in high risk patients with incremental costs considered acceptable from a U.S. perspective.

The largest prospective longitudinal study of acute myocardial infarction (MI) patients offers unique insight into the use, safety and effectiveness of blood thinners prasugrel and clopidogrel in hospitals across the United States.

September 19, 2014 — A new study investigating different durations of triple therapy for anticoagulation after drug-eluting stent (DES) implantation demonstrated that six weeks of drug therapy was not superior to six months of therapy with regard to net clinical outcomes. ISAR-TRIPLE is the largest randomized trial to date investigating triple therapy after stenting and the first trial evaluating the duration of triple therapy. Findings were reported earlier this week at the 2014 Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
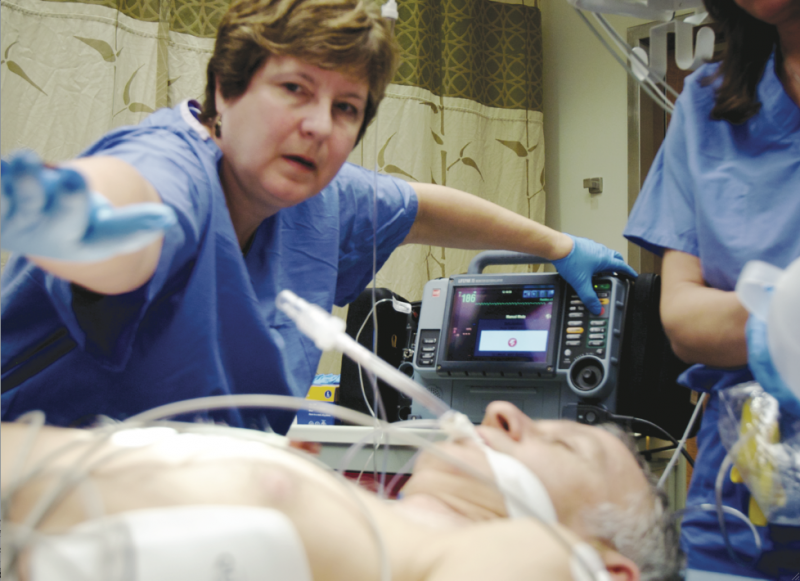
September 19, 2014 — According to a new study, peritoneal hypothermia is feasible for patients who have suffered ST-segment elevation myocardial infarction (STEMI). However, the procedure was associated with an increased rate of adverse events without reducing infarct size. These findings were reported at the 26th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium earlier this week.
New data from a landmark clinical trial found that after five years, transcatheter aortic valve replacement (TAVR) demonstrated a persistent mortality benefit, improved functional status, and resulted in a lower rate of repeat hospitalizations when compared with standard therapy for patients with severe aortic stenosis who are not candidates for surgery.
A new study found that fractional flow reserve (FFR)-guided provisional side branch (SB) stenting of true coronary bifurcation lesions yields similar outcomes to the current standard of care. The DKCRUSH-VI clinical trial is the first study to compare FFR-guided and angiography-guided stenting.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
September 17, 2014 — A new clinical trial found that vascular closure devices (VCDs) are non-inferior to manual compression in patients undergoing transfemoral coronary angiography. The findings were reported at the 2014 Transcatheter Cardiovascular Therapeutics (TCT) annual scientific sessions in Washington, D.C.
September 17, 2014 — GE Healthcare announced it has installed its Cath Lab Efficiency Manager at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. The solution is a new analytical tool that analyzes the performance of an interventional lab, providing hospitals with important data they can use to identify areas of improvement.
Unexpected trips to the hospital are inconvenient and worrisome for anyone, but for congestive heart failure sufferers, they can be all too frequent.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
BioControl Medical announced that it has reached an important clinical trial milestone, reaching 480 randomized subjects — or 70 percent — of the planned 650 subjects with congestive heart failure (HF) in the INOVATE-HF (INcrease Of VAgal TonE in Heart Failure) trial of its CardioFit system, the first medical device designed to treat HF using neurostimulation.
September 16, 2014 — A quarter of adults in the United States have two or more chronic medical conditions, as do more than two-thirds of seniors, yet there are few clinical practice guidelines for cardiologists that take such comorbid conditions and their treatment into consideration.
September 16, 2014 — Advanced Accelerator Applications (AAA), an international specialist in molecular nuclear medicine, announced this week it has entered into an agreement with GE Healthcare in Italy to acquire its Italian FDG-PET imaging agent business (fluorodeoxyglucose positron emission tomography). This acquisition includes the license to market GE Healthcare’s SteriPET (FDG) imaging agent in Italy.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Tryton Medical Inc. announced that the first patient in the United States has been enrolled in the Extended Access Registry, a single arm study of its Tryton Side Branch Stent.
The Minneapolis Heart Institute Foundation (MHIF) announced its first implant of the Portico Re-sheathable Transcatheter Aortic Valve System, developed by St. Jude Medical.
September 15, 2014 — A new gene-directed drug being tested through a research study called GENETIC-AF may improve care, quality of life and survival in patients with heart failure and atrial fibrillation (AF). A previous study showed the drug being evaluated, called bucindolol hydrochloride, reduced symptomatic AF when compared with placebo in patients with a specific gene variation, or genotype. In the current study, bucindolol, a betablocker, is being compared with another betablocker called metoprolol succinate, a heart failure drug approved by the U.S. Food and Drug Administration (FDA).

 September 19, 2014
September 19, 2014











