Health and Human Services Secretary Sylvia M. Burwell announced measurable goals and a timeline to move the Medicare program and the healthcare system at large toward paying providers based on the quality, rather than the quantity of care they give patients. The announcement was made in a meeting with nearly two dozen leaders representing consumers, insurers, providers and business leaders.

Three-dimensional (3-D) printing can effectively create a biodegradable tracheal segment containing a patient’s own cells for use in complex tracheal reconstruction, according to a proof of concept study abstract released at the 51st Annual Meeting of The Society of Thoracic Surgeons.
CytoSorbents Corporation announced that it has submitted an Investigational Device Exemption (IDE) application to the U.S. Food and Drug Administration (FDA) to conduct its proposed clinical trial using CytoSorb intra-operatively in patients undergoing complex cardiac surgery requiring the use of a heart-lung machine.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Ortho-Clinical Diagnostics Inc. announced the nationwide availability to hospitals of the Nephrocheck Test System designed to help healthcare providers identify patients at risk of developing moderate or severe acute kidney injury (AKI) within 12 hours of patient assessment.
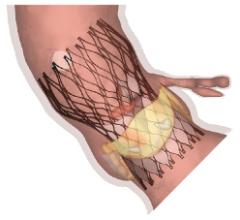
FEops announced the closing of a €1.3 million (~$1.9 million USD) series A financing round, led by Capricorn Venture Partners and PMV. The funding will be used to support the launch of FEops’ first product, TAVIguide, in key markets in Europe and the United States.

According to a study published online in the Journal of the American College of Radiology (JACR), large majorities of primary care physicians believe that advanced medical imaging, such as computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET) provides considerable value to patient care.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

The British Heart Foundation (BHF) announced the winners of its annual ‘Reflections of Research’ image competition – reflecting the charity’s research into heart and circulatory diseases.
CardioKinetix Inc. announced the completion of enrollment in Parachute China, a multi-center trial to evaluate the minimally invasive Parachute Ventricular Partitioning Device for the treatment of heart failure.
Kopp Development Inc. released a new entryway system — FerrAlert Halo II Plus.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
St. Jude Medical Inc. announced the first patient enrollment in the STAR-VT (Substrate Targeted Ablation using the FlexAbility Ablation Catheter System for the Reduction of VentricularTachycardia) clinical trial, a prospective, multi-center, randomized study evaluating the safety and efficacy of the FlexAbility ablation catheter in ventricular tachycardia (VT) ablation procedures.
Cleveland HeartLab (CHL) announced that it has acquired the MIRISK cardiovascular disease (CVD) risk assessment tool. Developed at Stanford University School of Medicine and validated in an eight-year, 5,000-patient clinical study, MIRISK is a highly accurate tool for determining a patient's potential long-term risk of a heart attack.
AstraZeneca announced that the PEGASUS-TIMI 54 study, a large-scale outcomes trial involving more than 21,000 patients, successfully met its primary efficacy endpoint. The study assessed ticagrelor (Brilinta) tablets at either 60 mg twice daily or 90 mg twice daily plus low-dose aspirin for the secondary prevention of atherothrombotic events in patients who had experienced a heart attack one to three years prior to study start. The primary efficacy endpoint was a composite of cardiovascular (CV) death, myocardial infarction (MI) or stroke.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Medtronic Inc. announced the first patient enrollment in the WRAP Infection Clinical Trial, which will evaluate the effectiveness of the TYRX Absorbable Antibacterial Envelope in reducing major infections in patients with cardiac implantable electronic devices (CIEDs) at risk for infection.
In a rural Maine county, sustained community-wide programs targeting cardiovascular risk factors and behavior changes were associated with reductions in hospitalization and death rates over a 40-year period (1970-2010) compared with the rest of the state. Substantial improvements were seen in control of hypertension, cholesterol and smoking cessation, according to a study in the January 13 issue of the Journal of the American Medical Association (JAMA).
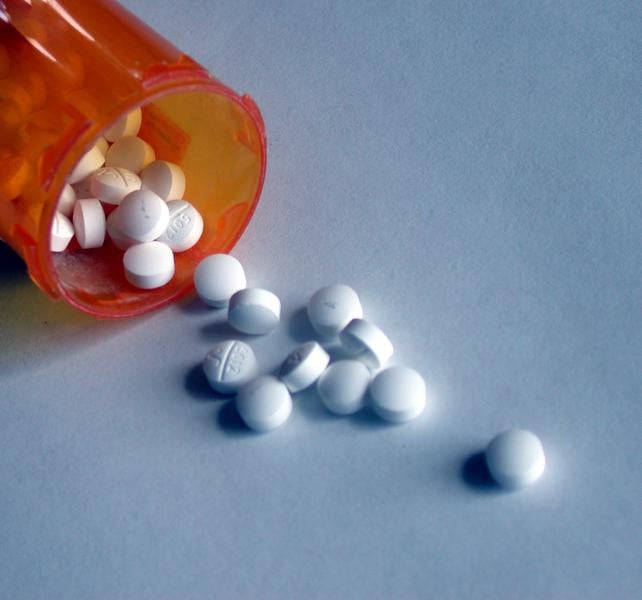
A new post-marketing study evaluating the safety of once-daily rivaroxaban (Xarelto) shows, in patients with non-valvular atrial fibrillation (NVAF), rates and patterns of major bleeding in routine clinical practice are generally consistent with those observed in Phase 3 clinical trials used to approve the medicine for this indication. These 15-month results, published in Clinical Cardiology, represent initial findings from an ongoing, five-year observational study of patients using rivaroxaban daily over the course of their lives.

 January 27, 2015
January 27, 2015