Coronary bypass graft surgery commonly used to treat patients with coronary heart disease results in improved quality of life but also costs more than another often-used less invasive technique, Christiana Care Health System researchers reported in January in the Journal of the American College of Cardiology.
A researcher in the University of the Basque Country’s (UPV/EHU) Department of Communications Engineering has developed the Ladon security protocol, an efficient mechanism to authenticate, authorize and establish the end-to-end keys (keys for communication between the terminal used by the doctor and the patient's device) for ICDs.
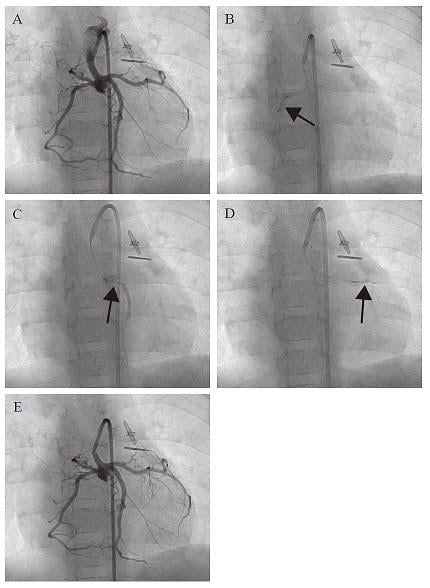
Researchers at Okayama University and Okayama University Hospital show that children suffering from a condition known as hypoplastic left heart syndrome experienced some improvement in cardiac function in the months following injection of cardiosphere-derived cells (CDCs).
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Healthcare Supply Chain Association (HSCA) President Curtis Rooney released a statement on the annual Budget and Economic Outlook compiled by the Congressional Budget Office (CBO).
AliveCor Inc. announced it has received CE Mark clearance for its automated analysis process (algorithm) to detect atrial fibrillation (AF). The latest version of the AliveECG app for users in the United Kingdom and Ireland now provides patients with real-time AF detection in electrocardiogram (ECG) recordings using the AliveCor Heart Monitor.
The British Medical Journal published results online that show Abbott’s Architect Stat High Sensitive Troponin-I (hsTnl) test may help doctors to detect twice as many myocardial infarctions (MI) in women. Results showed that Abbott’s test was able to diagnose MI in 22 percent of cases for women compared to a standard of 11 percent, when using a sex-specific threshold.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
In his lecture, Harrington will argue that simplification of larger trials, better early-phase investigations and the use of electronic health records can ease the path to new research.
Biosensors International Group has announced a distribution agreement with Veryan Medical Ltd. for BioMimics 3D, a nitinol stent with three-dimensional helical geometry designed for use in the superficial femoral artery.
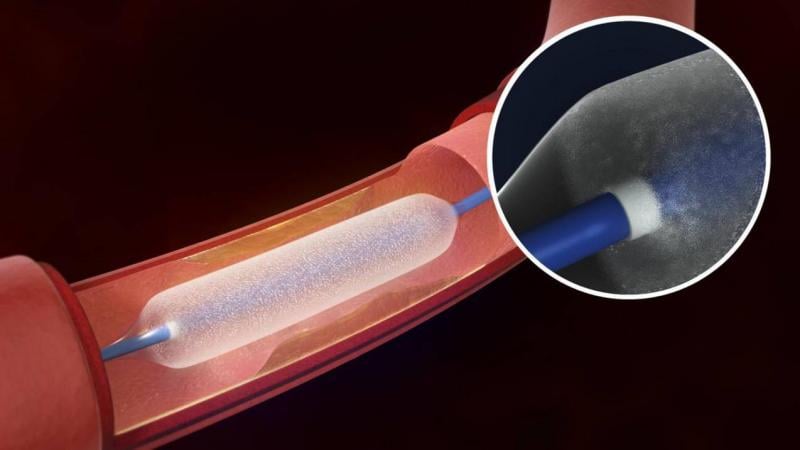
ECRI Institute has created a report that offers an overview of drug-eluting balloon (DEB) technology, “Health Technology Forecast report, "Drug-eluting Angioplasty Balloons for Preventing Restenosis after Revascularization." The report details some of the key questions regarding DEBs.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
SynCardia Systems Inc. has received U.S. Food and Drug Administration (FDA) approval to conduct a clinical study of the effectiveness of the SynCardia temporary Total Artificial Heart for permanent use, also called destination therapy.
The U.S. Food and Drug Administration (FDA) has approved for human evaluation a nanoparticle-based imaging agent jointly developed at Washington University School of Medicine in St. Louis and the University of California, Santa Barbara, in collaboration with Texas A&M University.
Medtronic announced new results from the PainFree SST and Shock-Less clinical studies published in the journal HeartRhythm.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Merge Healthcare Inc. announced that Merge Cardio has been named Best in KLAS for the cardiology software category in the "2014 Best in KLAS: Software & Services" report. In addition, Merge Hemo has been named the KLAS Category Leader in Cardiology Hemodynamics. This marks the second and fourth consecutive wins for Merge Cardio and Merge Hemo, respectively.
The 2014 Best in KLAS report ranks healthcare vendors and their solutions by the professionals who use them—healthcare providers.

There are several new interventional and minimally invasive surgical heart failure (HF) devices in development or in trials that might offer new ways to boost patient volume in the coming years. There is a lot of potential economic opportunity in new HF therapies, since HF represents the single-largest cause of hospitalizations in many countries and it is the largest expense in the U.S. Medicare budget.


 February 03, 2015
February 03, 2015













