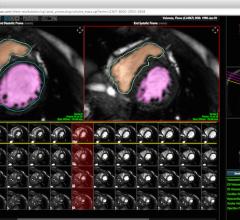
TomTec Imaging Systems GmbH introduced AutoStrain software for automated strain analysis and quantitative visualization of left ventricular function at the 64th Annual Scientific Session of the American College of Cardiology (ACC 2015) in San Diego.
The Centers for Medicare & Medicaid Services (CMS) is working closely with stakeholders across the healthcare industry to provide support in transitioning to version 10 of the International Classification of Diseases (ICD), including an online resource. CMS will officially transition from ICD-9 to ICD-10 on Oct. 1, 2015.

Tryton Medical Inc. announced that results from the company’s pivotal clinical trial for the Tryton Side Branch Stent were published in the Journal of the American College of Cardiology. The Tryton Pivotal IDE Trial, which enrolled 704 patients at 67 centers in North America and in 11 countries in the European Union (EU) and Israel, was the largest coronary bifurcation study ever conducted.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
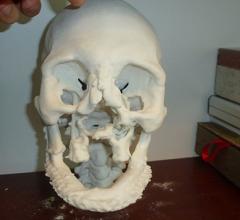
A study being presented at the Society of Interventional Radiology’s annual scientific meeting says 3-D printing could become a powerful tool in customizing interventional radiology treatments to individual patient needs.
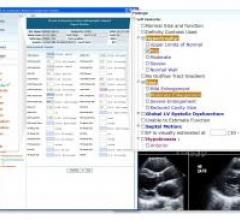
ScImage announced the immediate availability and deployment of updated quantification metrics based on guidelines published jointly in January 2015 by the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI).
Heart Imaging Technologies announced the release of Precession, a cardiac magnetic resonance solution which allows viewing, analysis and reporting all within a standard web browser.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

Johns Hopkins cardiologists report they have developed a formula that estimates one’s risk of dying over a decade based on a person’s ability to exercise on a treadmill at an increasing speed and incline.
New and updated American College of Radiology (ACR) Appropriateness Criteria now help healthcare providers choose the most appropriate medical imaging exam or radiation therapy for more than 1,000 clinical indications. These continually updated criteria are a national standard developed by expert panels of physicians from many different medical specialties.
CompView Medical (CVM) introduced an all-in-one equipment manager, visualization and ergonomic boom system — the NuCart.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

Frequent use of lead aprons in the interventional lab and radiology departments to protect against radiation exposure is associated with increased musculoskeletal pain, according to a study published in the Journal of the American College of Cardiology.

Cardinal Health announced plans to acquire Johnson & Johnson’s Cordis business, a leading global manufacturer of cardiology and endovascular devices, for $1.94 billion in cash.
In a new report, SmarTech Markets Publishing forecasts shipments of 3-D printers in the medical industry will reach 2,135 units in 2020, growing to 3,055 units in 2024. The report, "3-D Printing in Medical Markets: An Opportunity Analysis and Ten-Year Forecast," shows where opportunities will be found.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Hitachi Aloka Medical America Inc. announced the creation of a new dedicated sales force targeting cardiovascular ultrasound opportunities in physician’s offices and hospitals.
AirStrip and Life Monitor Pty Ltd. announced the formation of a strategic partnership to sell AirStrip solutions in Australia and New Zealand. Both the AirStrip ONE mobile interoperability platform and the Sense4Baby wireless fetal/maternal monitoring system will be available as part of the agreement.
The American Medical Society for Sports Medicine (AMSSM) and the FIFA Medical Assessment and Research Center (F-MARC) joined together to host the 2nd Summit on ECG Interpretation in Athletes, Feb. 26-27 in Seattle. The summit brought together top sports cardiology and sport medicine physicians from around the world to focus on improving cardiac safety in athletes and impacting sudden cardiac death.

 March 04, 2015
March 04, 2015