The current monitoring of patients with cardiac implantable electronic devices (CIEDs) may be underestimating device problems, according to UC San Francisco researchers. They propose systematic methods to determine accurate causes of sudden death in those with CIEDs such as defibrillators and pacemakers, as well as improved monitoring for device concerns.
The Medicines Company announced the approval of cangrelor (Kengreal) by the U.S. Food and Drug Administration (FDA) as an adjunctive therapy to percutaneous coronary intervention (PCI). The novel, intravenous antiplatelet agent is indicated for reducing periprocedural thrombotic events in patients who have not been treated with a P2Y12 inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor (GPI).
The American Society of Echocardiography (ASE) and Ke Labs jointly announced in May an agreement to co-develop and co-market a new cardiac ultrasound software application. The application is designed to ensure quality and consistency of cardiac ultrasound image review — both quantitatively and qualitatively — and inform and educate users on interesting cases, new techniques and other changes. This medical imaging quality management software tool is generally referred to as the “Echocardiography Test and Teach Application,†and it has been designed through the expert guidance of Harvey Feigenbaum, M.D., FASE with the Krannert Institute of Cardiology at Indiana University Health and founder of the ASE.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Epsilon Imaging Inc. announced that several studies were presented at the American Society of Echocardiography (ASE) 2015 conference, June 13-16, on its EchoInsight automated measurement suite for improved analysis and interpretation in echo.
Agfa HealthCare announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for diagnostic viewing with the XERO Viewer. This Full Fidelity View functionality of the viewer uses lossless compression to provide diagnostic quality when images are displayed, by retrieving original-quality renditions of stored digital X-ray (DX), computed tomography (CT), magnetic resonance imaging (MRI), computed radiography (CR) and ultrasound (US) images.
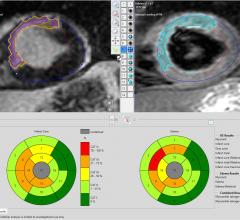
Pie Medical Imaging announced its new release of CAAS MRV, for analysis of the heart to support diagnosis of cardiovascular conditions. This new version contains intuitive and guided analysis workflows, optimized to clinical practice. The workflows guide users through the required steps for functional left and right ventricle, viability and first pass perfusion analysis. The new viability workflow includes, besides analysis of delayed enhanced MR (magnetic resonance) images, also analysis of edema on T2-weighted MR images. The combination of segmented infarcts and edema areas can identify the salvageable areas.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
ClearFlow Inc. announced that the U.S. Food & Drug Administration (FDA) has granted expanded Indications for Use of the company's patented PleuraFlow Active Clearance Technology system. The PleuraFlow system is a patented medical device that prevents chest drains from occluding with clot, which can lead to retained blood around the heart and lungs.
Edwards Lifesciences Corp. announced U.S. Food and Drug Administration (FDA) approval of the Edwards Sapien 3 valve with the Commander Delivery System for the treatment of high-risk patients suffering from severe, symptomatic aortic stenosis.
ERT announced the integration of a wearable biosensor which captures and wirelessly transmits real-time, continuous, clinical-grade biometric measurements into the ERT electronic Clinical Outcome Assessment (eCOA) system. ERT showcased a proof-of-concept demonstration of the integration at its Innovation Lab at the Drug Information Association (DIA) Annual Meeting, June 14-17, in Washington, D.C.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
GE Healthcare announced the launch of its new introductory dose management solution, DoseWatch Explore, which is now available on select GE computed tomography (CT) systems in the United States. DoseWatch Explore is a Web-based, cloud-deployed, dose management software that tracks, analyzes and reports practice-level radiation dose data for GE CT systems.
Calgary Scientific Inc. announced the release of ResolutionMD 5.1. The latest software version enables physicians to make faster and more informed treatment decisions, by providing even greater access to medical images and information via Web and mobile devices.
Atrial fibrillation patients on the anticoagulant dabigatran were more likely to adhere to the medication with appropriate patient selection and pharmacist-led monitoring, according to a study in the April 14 issue of JAMA. The study looked at patients who filled dabigatran prescriptions at Veterans Health Administration (VHA) sites, and found variability in patient medication adherence across sites.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Stentys announced in April that it received CE Marking for its new Self-Apposing stent system ahead of plan. The designation will allow the company to market the new products in Europe and in all the other countries where this certification is recognized. The Xposition S stent officially launched at the EuroPCR conference on May 19, 2015.
IMRIS Inc. received a letter May 26 from the NASDAQ Stock Market stating that it would be delisted and trading suspended on June 4. IMRIS said this was in accordance with Listing Rules 5101, 5110(b), and IM-5101-1. A Form 25-NSE will be filed with the Securities and Exchange Commission (the SEC), which will remove the company's securities from listing and registration on The Nasdaq Stock Market.
Toshiba America Medical Systems Inc. has received U.S. Food and Drug Administration (FDA) clearance for detector and computed tomography (CT) software enhancements for its Aquilion ONE family of CT systems. which are designed to offer customers the right technology to meet their business needs for improving patient safety, have received FDA clearance.

 June 22, 2015
June 22, 2015