Merit Medical Systems Inc. announced the launch of the Prelude Snap splittable sheath introducer.
GE Healthcare introduced a new in-field upgrade program for 1.5T magnetic resonance imaging (MRI) systems in most regions globally. A currently installed GE Healthcare 1.5T LCC magnet may be upgraded to the new Signa Explorer Lift so customers can benefit from the modernized patient comfort, workflow efficiency and diagnostic quality of this system. By upgrading, customers can potentially benefit from cost savings in multiple ways: up to 50 percent savings in construction cost, up to 50 percent savings in equipment cost in comparison to a new 1.5T system, and up to 30 percent increase in procedures due to potential increased throughput and referrals.
Celebrating its 25th anniversary, Materialise announces the launch of the latest Mimics Innovation Suite, including the Mimics 18.0 and 3-matic 10.0 software solutions. The new and improved tools increase user-friendliness, reduce segmentation time and make design and modeling even more realistic. It’s also possible to 3-D print the results in full color. In addition, the visualization capabilities have been expanded with a fluoroscopy view and virtual X-ray simulation.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

The Accreditation for Cardiovascular Excellence (ACE) is the exclusive international provider of interventional cardiology, electrophysiology, congenital heart disease and endovascular catheterization lab accreditation, and a valuable resource for asserting a commitment to the highest standards of care in invasive cardiology. Since 2011, (ACE) has reviewed the structure, internal processes, patient safety practices, and clinical outcomes of cardiac catheterization laboratories. To achieve ACE accreditation, facilities must demonstrate compliance with rigorous ACE quality standards that are based on published guidelines, expert consensus documents, and scientific data.
Non-calcified arterial plaque is associated with diabetes, high systolic blood pressure and elevated “bad” cholesterol levels in asymptomatic individuals, according to a new study published online in the journal Radiology.
The Valley Hospital has been selected as the United States Coordinating Center for the international PRAETORIAN trial analyzing implantable cardioverter-defibrillators (ICDs). The trial will compare traditional ICDs with a newer model that may reduce the risk of complications associated with these otherwise life-saving devices.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
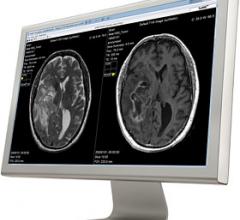
Cincinnati Children’s Hospital Medical Center (CCHMC) has evaluated synthetic magnetic resonance imaging (MRI) on pediatric cases to study and clinically validate its use on children. Results from the first part of the evaluation, which used SyMRI software, shows the synthetic images are diagnostically satisfactory in comparison with conventional sequences.

According to a new report from BCC Research, the global market for disposable medical sensors grew to $4.2 billion in 2014 from $3.8 billion in 2013. The market is expected to grow at a five-year compound annual growth rate (CAGR) of 10.2 percent from 2014 to 2019, reaching $6.8 billion in 2019.

Long-term exposure to ionizing radiation is a primary occupational concern for today's interventional cardiologists. To address these concerns, many interventional cardiologists are enlisting the support of their hospital administrators to create workplace environments that reduce exposure to ionizing radiation.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Two all touch-screen interface Carestream Touch Ultrasound Systems have received U.S. Food and Drug Administration (FDA) 510(k) clearance and are now commercially available in the United States. The Touch Prime Ultrasound System and the Touch Prime XE Ultrasound System are both premium systems designed for general diagnostic imaging use in radiology.

Maquet Getinge Group announced the publication of a manuscript describing the exploration of the hemodynamic effects of the newer, larger-capacity 50 cc intra-aortic balloon pumps (IABPs) versus 40 cc IABPs in real-world clinical practice. The paper, titled "Hemodynamic Effects of Standard Versus Larger-Capacity Intraaortic Balloon Counterpulsation Pumps," appears in the April 2015 volume of The Journal of Invasive Cardiology.
Silk Road Medical Inc. announced the company received U.S. Food & Drug Administration (FDA) 510(k) clearance for its Enroute transcarotid neuroprotection system (NPS). The Enroute transcarotid NPS is used to directly access the common carotid artery and initiate high rate temporary blood flow reversal to protect the brain from stroke while performing carotid angioplasty and stenting (CAS).
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...

Mitralign shared six-month data on its Mitralign Percutaneous Annuloplasty System (MPAS) for treatment of functional mitral regurgitation (FMR) at EuroPCR 2015 in Paris. The prospective, multi-center, single-arm study met its safety endpoint at 30 days and its performance endpoint at six months. In the clinical study, the MPAS demonstrated a statistically significant reduction in left ventricular diameter, significant reduction of both the A-P and S-L annular dimensions, and a significant improvement of the patient’s walking distance. The Mitralign Percutaneous Annuloplasty System is not approved for sale or distribution; however it is anticipated to receive CE marking in 2015.
National Security Technologies LLC (NSTec) and Henderson, Nevada-based Global Medical Isotope Systems (GMIS) announced a public-private partnership agreement for research and development of an essential radioactive isotope used in millions of medical diagnostic imaging procedures annually.
Agfa HealthCare announced the launch of its Enterprise Imaging Exchange Program, which establishes a secure health information exchange network between multispecialty collaborating health providers to exchange and share medical imaging data. The solution will be on display at the Society for Imaging Informatics in Medicine (SIIM) annual meeting, May 26-31, 2015, in Washington, D.C.


 June 03, 2015
June 03, 2015