ContextVision AB announced the development of a prototype technology that enables ultrasound imaging to be automatically optimized for each patient.
Medinol U.S. recently opened their commercial business in Parsippany, New Jersey, followed by the launch of the NIRxcell CoCr Stent System.
BioVentrix Inc. has achieved the first implantation of its micro-anchor heart failure (HF) technology from entirely within the left ventricle (LV) using a catheter-based endovascular approach.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
December 9, 2015 — During RSNA 2015, Cerner exhibited the latest version of its PowerChart Cardiovascular solution ...

Scientists have confirmed the role of a set of gene mutations in the development of congenital heart disease and simultaneously discovered a link between them and some neurodevelopmental abnormalities in children. These abnormalities include cognitive, motor, social and language impairments.
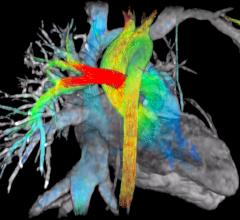
Arterys Inc. launched the Arterys System, an intelligence platform designed to enhance medical imaging, at the 101st Scientific Assembly and Annual Meeting of the Radiological Society of North America (RSNA), Nov. 29-Dec. 3 in Chicago.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
The eighth Image Wisely Radiation Safety Case — C-arm Based Cone Beam CT in Interventional Radiology — is now available to help radiologists, imaging technologists and medical physicists assess their understanding of important radiation safety concepts — including dose monitoring and optimization.
Researchers from the University of Toronto have found that a specific cell type plays a key role in maintaining healthy arteries after inflammation.
At Ahuja Medical Center, University Hospitals (UH) cardiologists recently implanted the Boston Scientific Emblem Subcutaneous Implantable Cardioverter-Defibrillator (S-ICD) system, the first such procedure done at this hospital.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Biotronik announced the first patient enrollments to the B3 clinical trial. The study will evaluate the potential clinical benefit of the physiologic rate response sensor Closed Loop Stimulation (CLS) in atrial fibrillation (AF) patients.
December 3, 2015 — Clinically viable model-based iterative reconstruction is now a reality with the launch of Toshiba ...
Feeling high levels of distress, fear and hostility prior to undergoing an angioplasty or other interventional radiology procedure may lead to a poor outcome, according to new research presented today at the annual meeting of the Radiological Society of North America (RSNA).
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Philips announced IntelliSpace Portal 8.0, the latest edition of its advanced data sharing, analytics and visualization platform that helps radiologists detect, diagnose and follow up on treatment of diseases.

December 2, 2015 — Researchers in the Netherlands studying thousands of healthy adults have found a connection between ...

December 2, 2015 — Researchers using modern imaging techniques on hearts more than 400 years old found at an ...


 December 09, 2015
December 09, 2015