January 7, 2016 — Celyad announced Dec. 21 that the U.S. Food and Drug Administration (FDA) has authorized the company’s ...
Roxwood Medical Inc. reported that more than 500 patients have been successfully treated as part of the initial limited release of its MicroCross Catheter, the latest addition to Roxwood Medical’s expanding product line. The MicroCross Catheter is U.S. Food and Drug Administration (FDA)-cleared and offered in two sizes (Micro14 and Micro18) for use in the coronary and peripheral vasculatures, providing enhanced guidewire support through challenging and tortuous anatomy.
Stroke is the third leading cause of death in the Western industrialized world, and according to the Centers for Disease Control (CDC), accounts for one of every 19 deaths in the United States each year. Over 80 percent of strokes are ischemic in nature, caused by clots and other obstructions in vessels that supply blood to the brain, and cardiac embolism accounts for approximately one-third of all cases of ischemic stroke. Echocardiography in general and transesophageal echocardiography in particular are essential for evaluation, diagnosis and management of stroke and systemic embolism. A new document, Guidelines for the Use of Echocardiography in the Evaluation of a Cardiac Source of Embolism, will appear in the January 2016 issue of the Journal of the American Society of Echocardiography (JASE) and provides the first set of guidelines from the American Society of Echocardiography (ASE) specific to this topic.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
January 7, 2016 — Scientists have found that women who suffer unexplained heart failure towards the end of pregnancy or ...
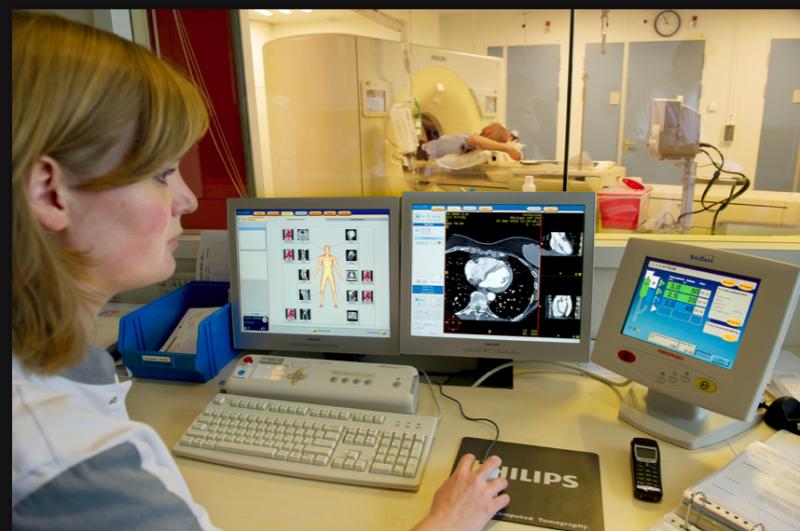
Corindus Vascular Robotics Inc. announced the launch of a CorPath robotic-assisted coronary intervention program at Massachusetts General Hospital (MGH) and the installation of its first CorPath System.
Clinical decision support (CDS) software has been discussed for years as a way to help clinicians follow best practice ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
A new study determined that the Watchman left atrial appendage closure device is more cost-effective than warfarin and non-warfarin oral anticoagulants (NOACs) for stroke reductions in non-valvular atrial fibrillation patients.
The Medicines Company announced Dec. 18 it has entered into a purchase agreement pursuant to which certain subsidiaries of Mallinckrodt plc will acquire The Medicines Company’s global portfolio of hemostasis products.
A new study from the Healthcare Information and Management Systems Society (HIMSS) offers insight from nearly 200 healthcare executives (C-Suite, administrators, directors and vice presidents) on their population health initiatives, and their current and future approach to population health IT solutions and consultants.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Johns Hopkins has demonstrated in animals that applying a pacemaker’s mild electrical shocks to push the heart in and out of normal synchronized contraction for part of each day may be an effective way to slow down the progression of heart failure.
Booz Allen Hamilton and Kaggle announced that the second annual Data Science Bowl will call on the global data science community to create a set of steps, or algorithms, to help transform diagnosis of heart disease. Through a partnership with the National Institutes of Health (NIH), participants in the 90-day competition will be given magnetic resonance imaging (MRI) images and asked to develop an algorithm to automate the measurements that are key indicators of heart disease.
January 4, 2016 — In a comparison of bleeding complications and mortality between transradial and transfemoral stent ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
January 4, 2016 — Living with pulmonary arterial hypertension (PH) is challenging, but the chore of treating the rare ...
December 31, 2015 — The U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel of the Medical Devices ...
December 31, 2015 - The U.S. Food and Drug Administration (FDA) has cleared Biotronik's Astron Peripheral Self-Expanding ...

 January 07, 2016
January 07, 2016













